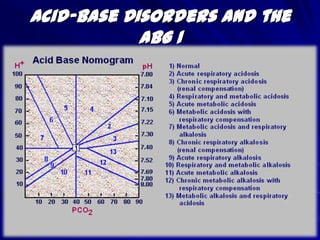
The document provides an overview of acid-base disorders and interpreting arterial blood gases (ABGs). It discusses the Henderson-Hasselbalch equation, compensation mechanisms, normal ABG ranges, and a step-by-step approach to analyzing ABGs. This includes checking the pH, pCO2, selecting the appropriate compensation formula, evaluating anion gap, delta-delta ratio, and urine pH to differentiate primary from combined acid-base disorders. Several case examples are then presented and analyzed using this approach.







![HCO3 [calculated vs measured]Oxygenation InformationPO2 [oxygen tension]](https://image.slidesharecdn.com/abg1-1317751311-phpapp01-111004130401-phpapp01/85/ABG1-SERIES-12-320.jpg)
![SO2 [oxygen saturation]Normal RangesPaO2 >80mm HgIn supine posture PaO2=109-(0.43 Х age)PaO2=100mmHg in 10 year old child, PaO2 falls approximately 5mmHG for every 10 years upto 90 years.5 mmHg higher in the sitting position that supine position](https://image.slidesharecdn.com/abg1-1317751311-phpapp01-111004130401-phpapp01/85/ABG1-SERIES-13-320.jpg)
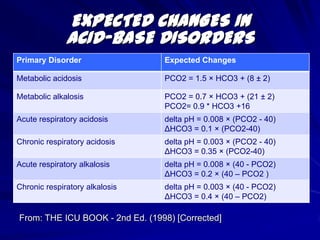


![EXPECTED CHANGES IN ACID-BASE DISORDERS From: THE ICU BOOK - 2nd Ed. (1998) [Corrected]](https://image.slidesharecdn.com/abg1-1317751311-phpapp01-111004130401-phpapp01/85/ABG1-SERIES-17-320.jpg)


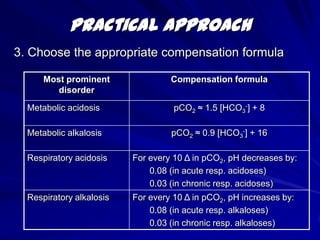






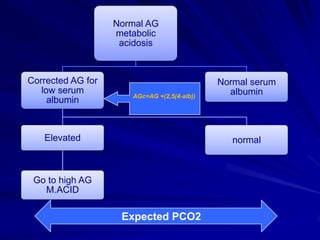
![Practical Approach5. Calculate the anion gap Anion gap = [Na+] – ( [Cl-] + [HCO3-] )If the anion gap is elevated, an elevated gap metabolic acidosis is likely present.](https://image.slidesharecdn.com/abg1-1317751311-phpapp01-111004130401-phpapp01/85/ABG1-SERIES-29-320.jpg)
![Practical Approach6. If an elevated gap acidosis is present, calculate the delta-delta ratio, to determine if a second metabolic disorder is present.Delta–Delta = Measured anion gap – Normal anion gap Normal [HCO3-] – Measured [HCO3-]](https://image.slidesharecdn.com/abg1-1317751311-phpapp01-111004130401-phpapp01/85/ABG1-SERIES-30-320.jpg)












![Case 4A 6 year old girl with severe gastroenteritis is admitted to the hospital for fluid rehydration, and is noted to have a high [HCO3-] on hospital day #2. An ABG is ordered:ABG: pH 7.47 Chem 7: Na+ 130 PCO2 46 K+ 3.2 HCO3- 32 Cl- 86 PO2 96 HCO3- 33Urine pH: 5.8](https://image.slidesharecdn.com/abg1-1317751311-phpapp01-111004130401-phpapp01/85/ABG1-SERIES-45-320.jpg)


