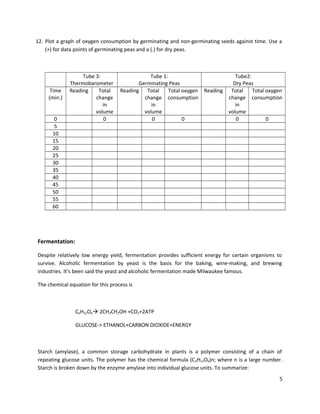
This lab aims to study aerobic respiration and alcoholic fermentation through experiments with seeds and yeast. Students will measure oxygen consumption of germinating and ungerminated pea seeds over 60 minutes using a volumeter. They will also observe gas production from yeast fermenting glucose, starch, and starch treated with amylase. The document provides background on metabolism, respiration, ATP, aerobic respiration, and fermentation. It outlines the objectives, materials, procedures, and data table for the oxygen consumption experiment and fermentation demonstration.

![within the bonds of carbohydrates is transferred to ATP during respiration. This stored energy can
be released later to power a wide variety of cellular reactions.
For aerobic respiration, the general equation is
C6H12O6 + 6O2 6CO2 +6H2O + 36 ATP+
Glucose + oxygen carbon dioxide +water+ chemical energy
If glucose is completely broken down to CO 2 and H2O about 686,000 calories of energy are released.
Each ATP molecule produced represents about 7500 calories of usable energy. The 36 ATP represent
270,000 calories of energy (36 x 7500 calories). Thus, aerobic respiration is about 39% efficient
[(270,000/686,000) x 100%].
By contrast, fermentation yields only 2 ATP. Thus, these processes are only about 2% efficient
[(2x7500/686,000) x 100%]. Obviously, breaking down carbohydrates by aerobic respiration gives a
bigger payback than the other means.
During the process of aerobic respiration, relatively high- energy carbohydrates are broken down in
stepwise fashion, ultimately producing the low-energy products of carbon dioxide and water and
transferring released energy into ATP. But what is the role of oxygen?
During aerobic respiration, the carbohydrate undergoes a series of oxidation-reduction reactions.
Whenever one substance is oxidized (loses electrons), another must be reduced (accept, or gain,
those electrons), another must be reduced (accept, or gain, those electrons). The final electron
acceptor in aerobic respiration is oxygen. Tagging along with the electrons as they pass through the
electron transport process are protons (H +). When the electrons and protons are captured by
oxygen, water (H2O) is formed:
2H+ + 2e- + ½ O2 H2O
In the following experiment, we examine aerobic respiration in two sets of seeds.
Oxygen Consumption:
One set of pea seeds has been soaked in water for the past 48 hours to initiate germination. In this
section, you will measure oxygen consumption to answer the question, “Do germinating peas have a
higher respiratory rate than ungerminated peas?”
Materials
•
Volumeter
2](https://image.slidesharecdn.com/6respiration-energyconversion-140122205457-phpapp02/85/6-respiration-_energy_conversion-2-320.jpg)




![yeast
+amylase
To calculate the volume of gas evolved, use the following equation: V = πr 2h, where π =3.14, r=
radius of tail of fermentation tube [r=1/2d (diameter)], h=distance from top of tail to level of
solution.
Did your results conform to your predictions? If not, speculate on reasons why this might be so?
________________________________________________________________________________
__________________________________________________________________________
What gas accumulates in the tail portion of the fermentation tube?
Questions:
1. If you performed the experiment on oxygen consumption without adding KOH pellets to the test
tubes, what results would you predict? Why?
2. Sucrose (table sugar) is a disaccharide composed of glucose and fructose. Glycogen is a
polysaccharide composed of many glucose subunits. Which of the following fermentation tubes
would you expect to produce the greatest gas volume over a 1-hour period? Why?
Tube 1: glucose plus yeast
Tube 2: sucrose plus yeast
Tube 3: glycogen plus yeast
8](https://image.slidesharecdn.com/6respiration-energyconversion-140122205457-phpapp02/85/6-respiration-_energy_conversion-8-320.jpg)

