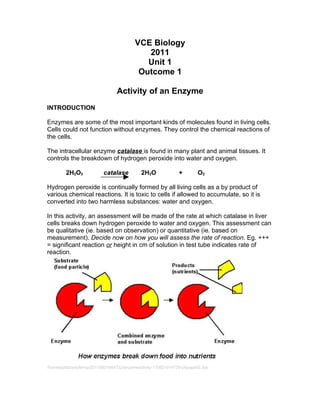
1. The experiment investigated factors that influence the rate of a chemical reaction catalyzed by the enzyme catalase. Liver tissue and hydrogen peroxide were used to test the effects of grinding, temperature, and controls.
2. Grinding the liver tissue increased the rate of reaction compared to whole tissue, likely by increasing the surface area exposed to hydrogen peroxide.
3. Boiling the liver tissue reduced its catalytic activity, probably by denaturing the catalase enzyme.