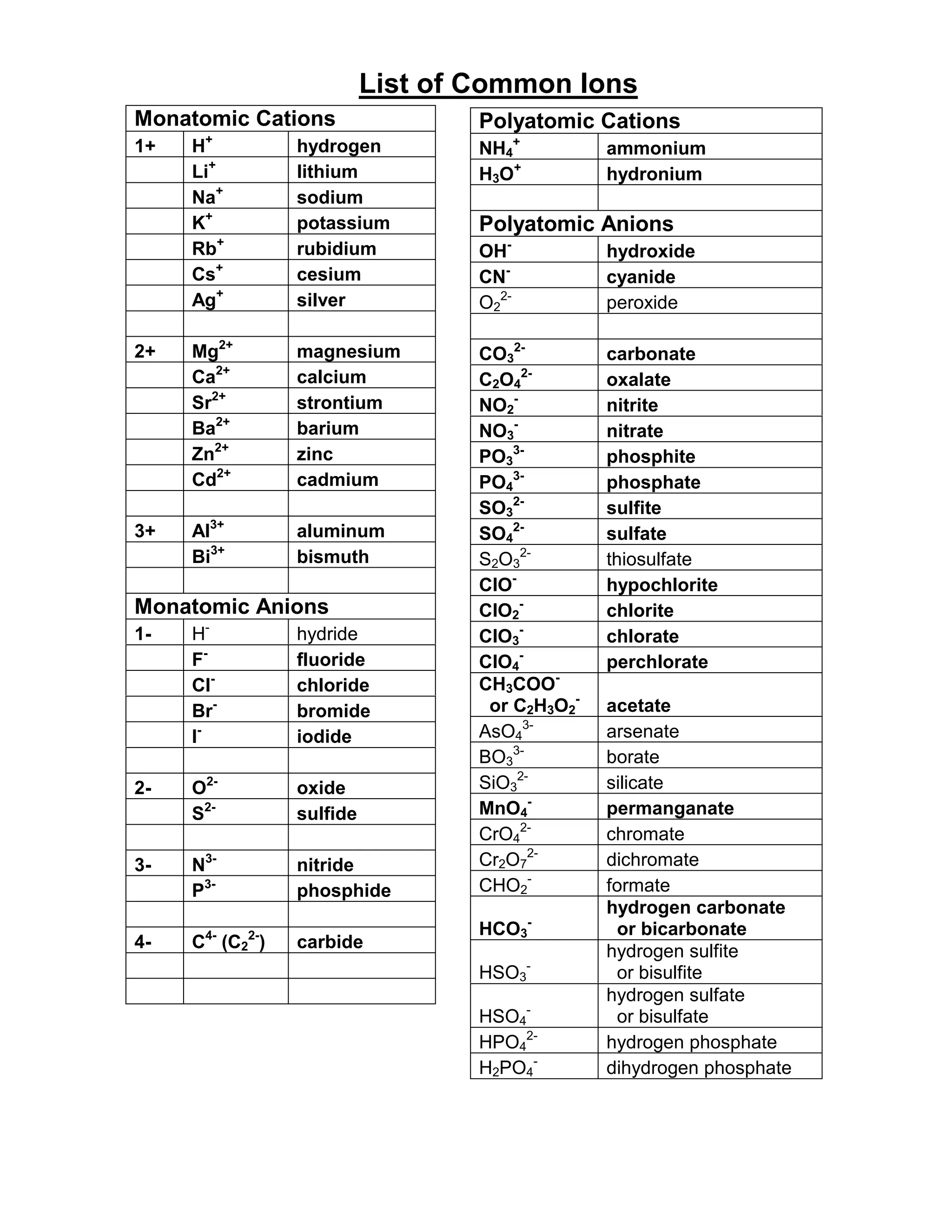
This document lists common ions found in chemistry. It includes monatomic cations such as hydrogen, lithium, sodium, and silver ions. Polyatomic cations include ammonium and hydronium ions. Monatomic anions include fluoride, chloride, bromide, and iodide ions. Polyatomic anions include carbonate, nitrate, phosphate, and sulfate ions. It also lists metals that can form multiple ions such as chromium, cobalt, copper, iron, lead, manganese, mercury, and tin.