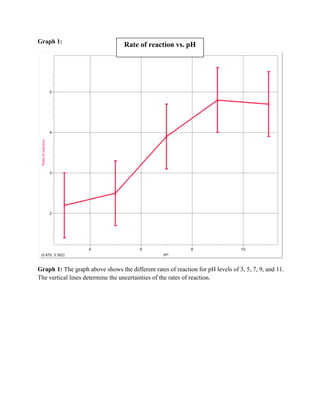
This experiment tested the relationship between catalase and hydrogen peroxide in different pH levels. Catalase is an enzyme that breaks down hydrogen peroxide, and its activity was measured by the rate of oxygen gas production, as indicated by changes in pressure. The results showed that reaction rates generally increased with higher pH levels up to pH 9, but continued rising past pH 7, contrary to expectations since catalase denatures above pH 7. Methodological weaknesses and unreliable pressure sensors may have impacted the accuracy of the results.