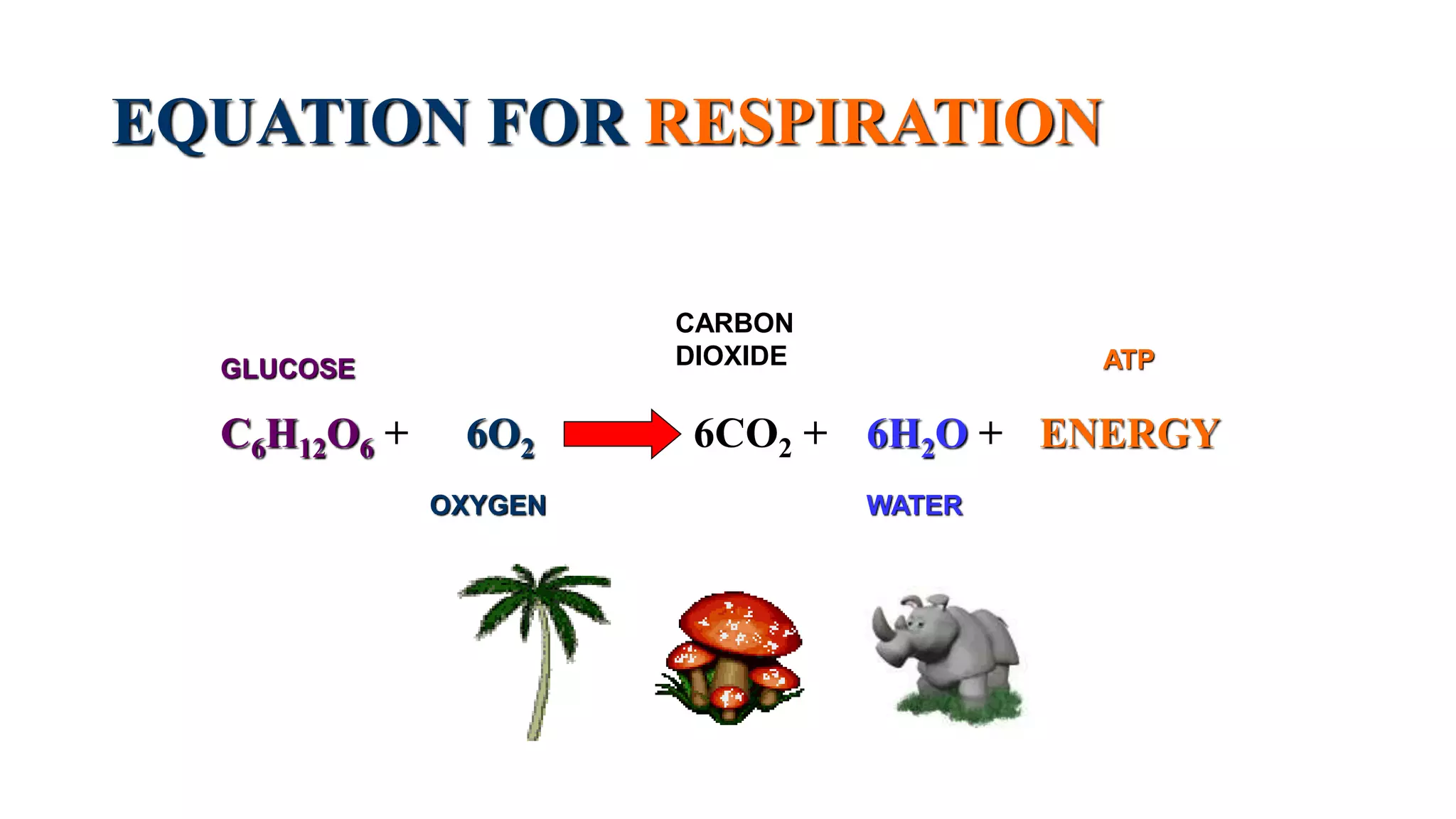
Photosynthesis and respiration are important chemical reactions that occur in plants. Photosynthesis uses carbon dioxide, water, and sunlight to produce glucose and oxygen. The equation for photosynthesis is: 6CO2 + 6H2O + Energy → C6H12O6 + 6O2. Cellular respiration breaks down glucose and uses oxygen to produce carbon dioxide, water, and ATP (energy). The equation for respiration is: C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy. In plants, photosynthesis occurs during the day while respiration occurs both day and night, with the two processes balancing the intake and output of gases.