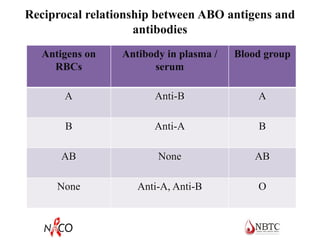
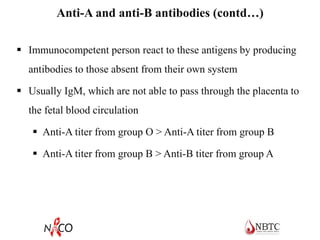
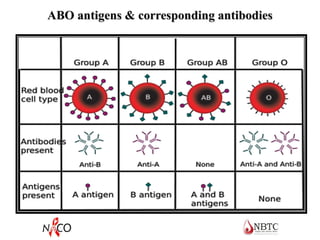
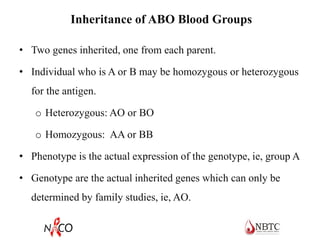
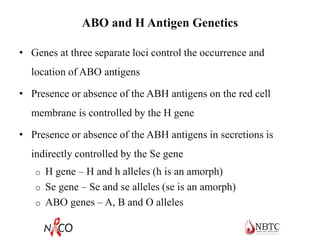
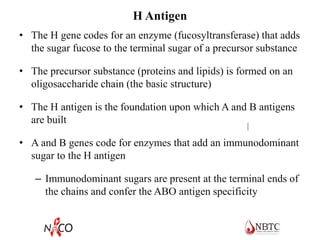
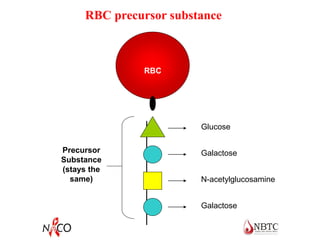
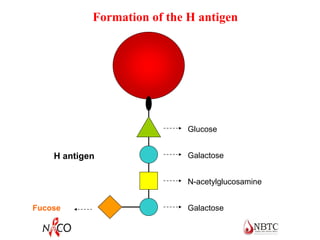
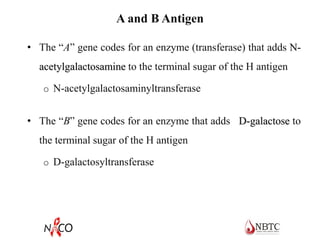
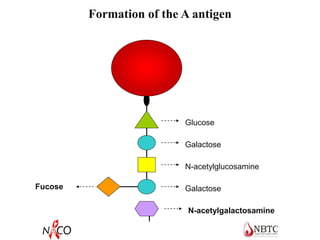
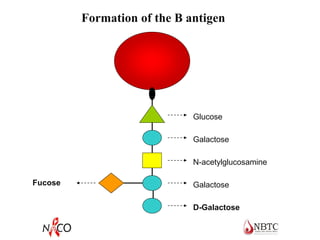
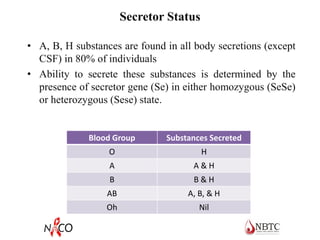
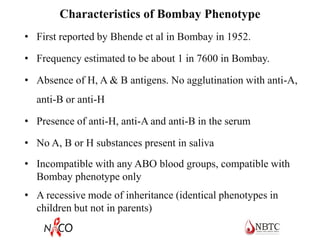
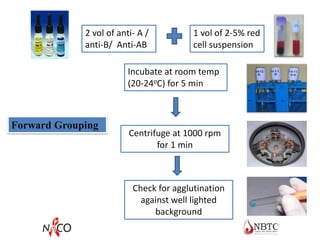
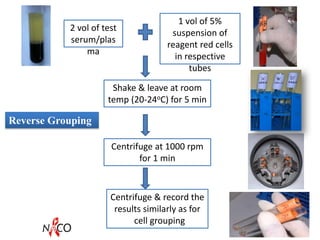
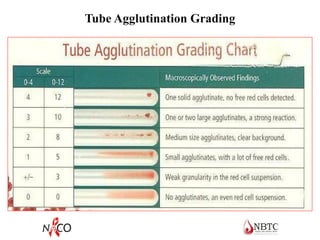
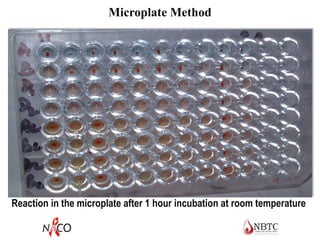
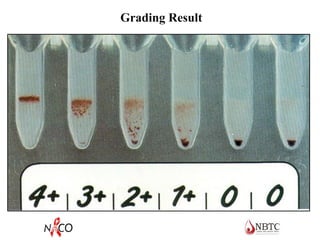
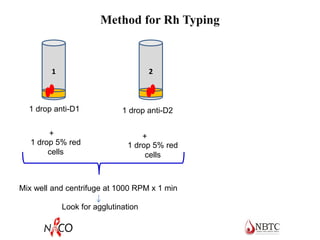
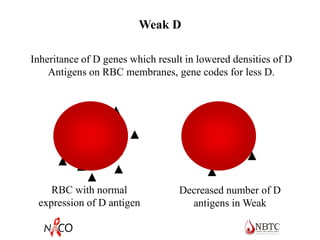
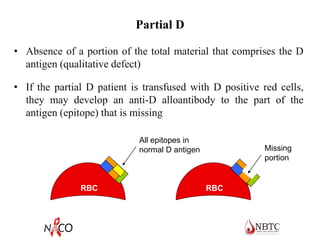
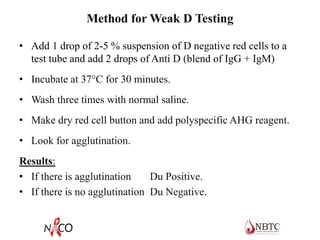
This document provides information about ABO and Rh blood grouping and typing. It discusses the antigens and antibodies involved in these two major blood group systems, including their inheritance, development, expression, and clinical significance. The key points covered include the reciprocal relationship between ABO antigens and antibodies, development of ABO antigens at birth, subgroups within the ABO system, inheritance of ABO blood groups, anti-A and anti-B antibody production, and Rh (D) antigen properties and typing. Practical aspects of ABO and Rh blood grouping using tube, gel, and microplate methods are also described.