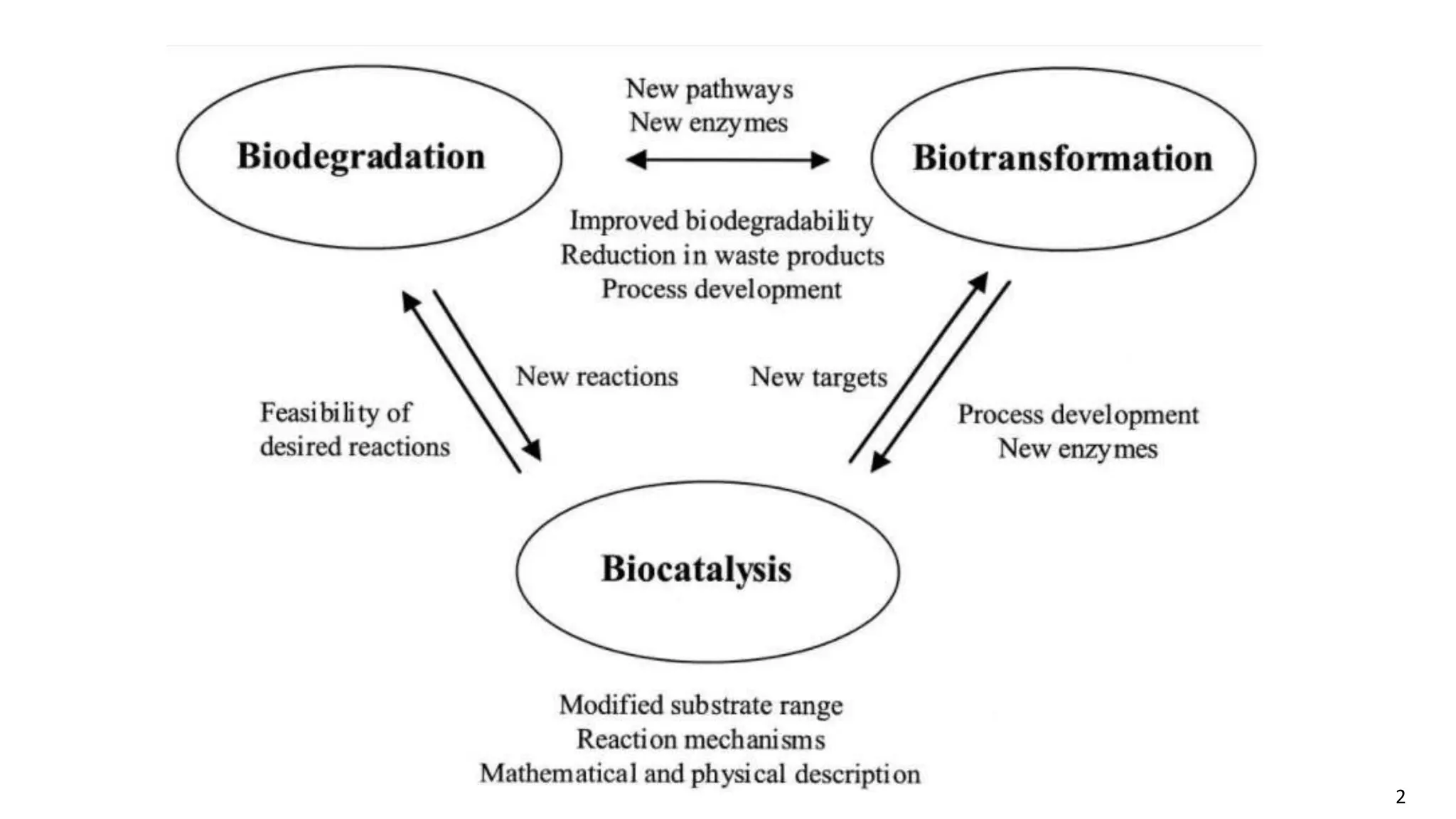
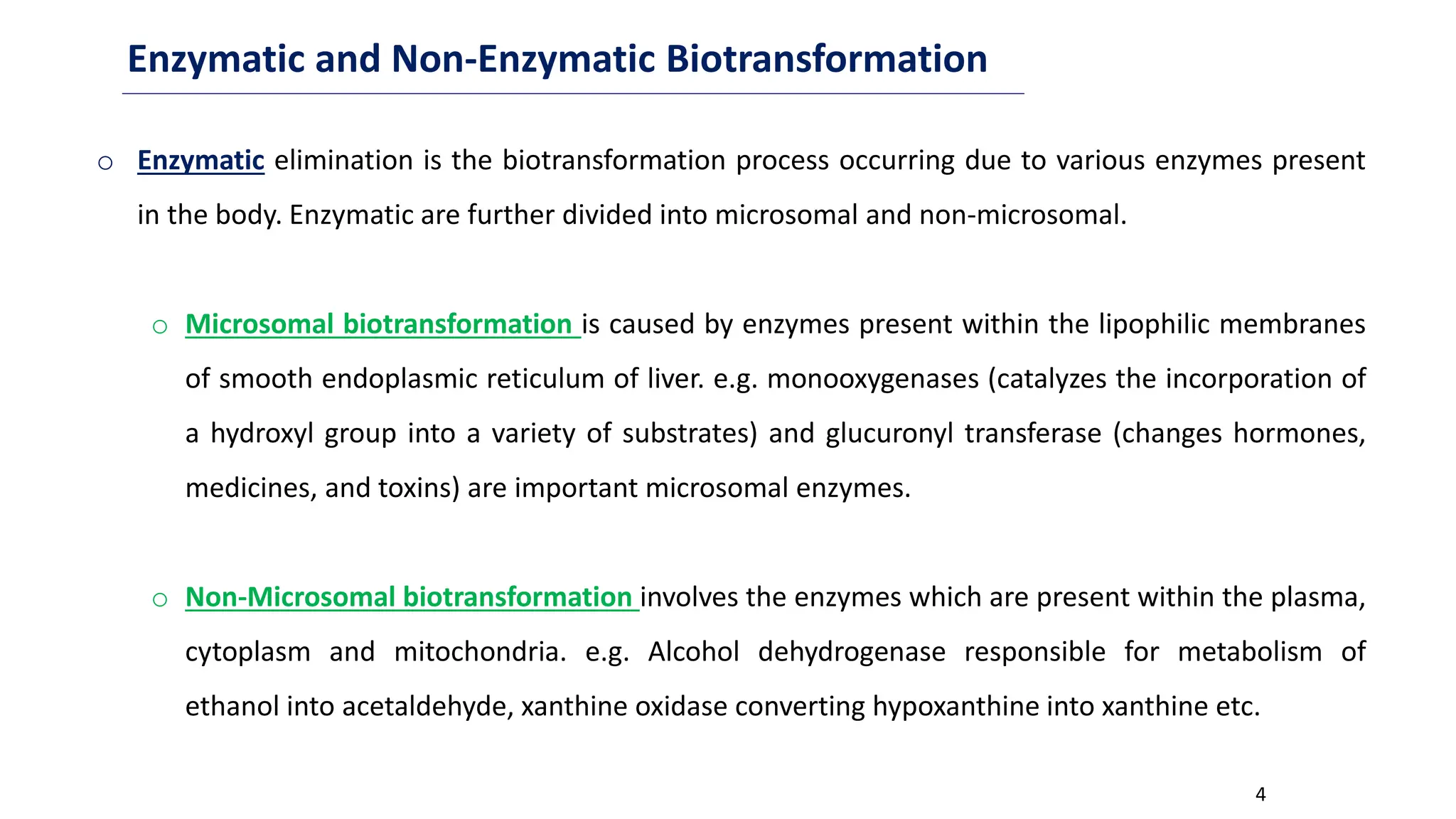
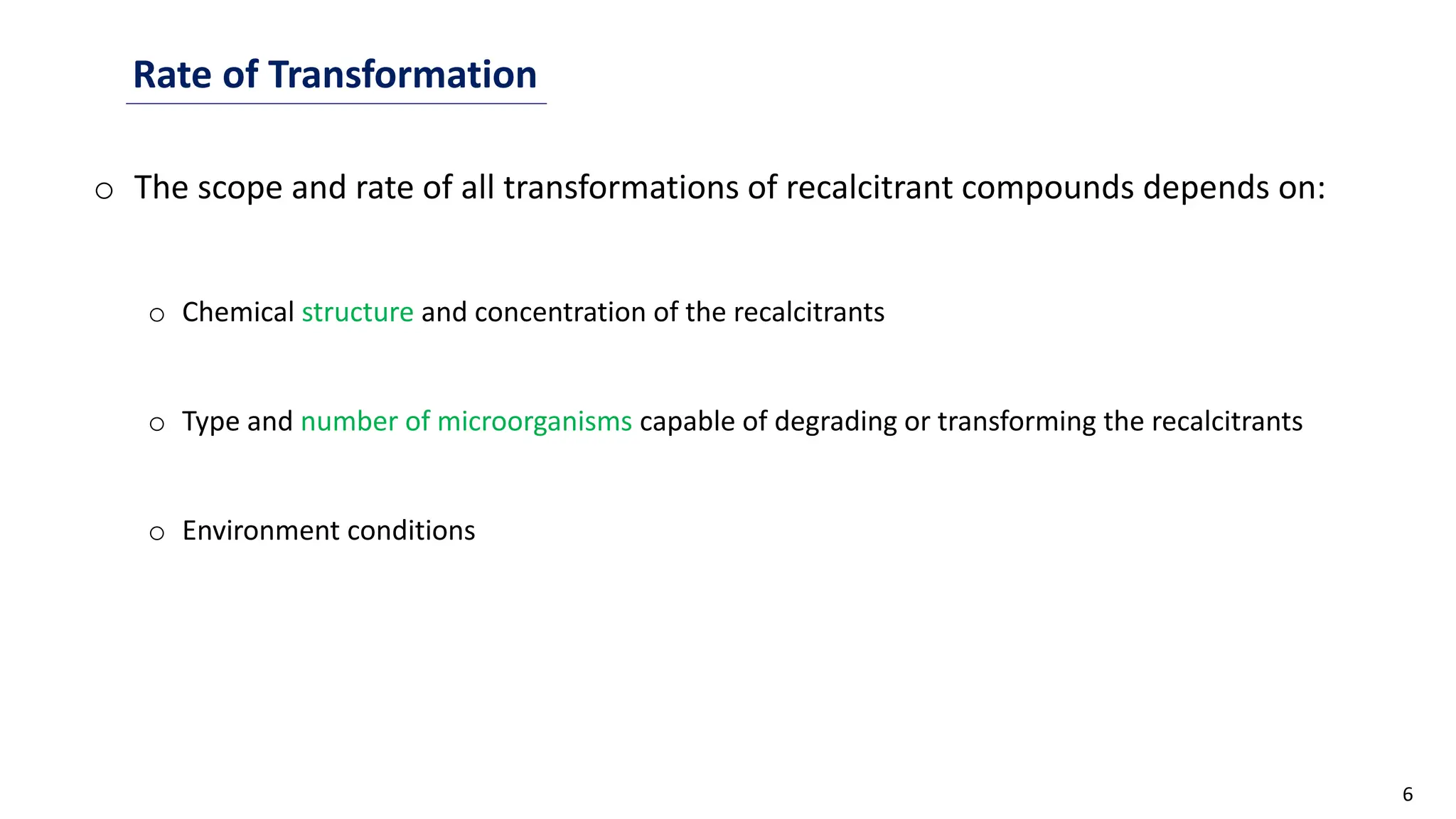
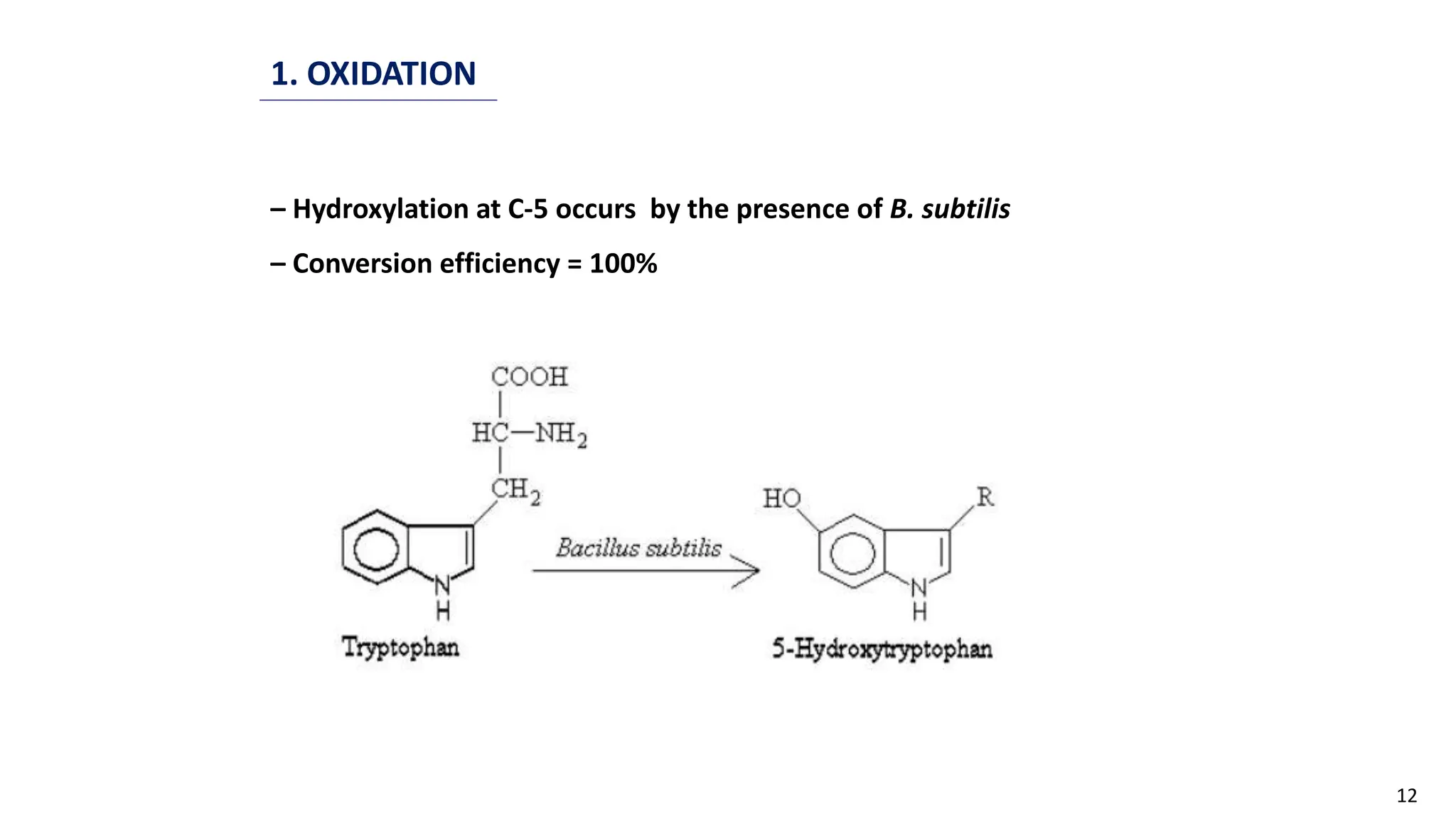
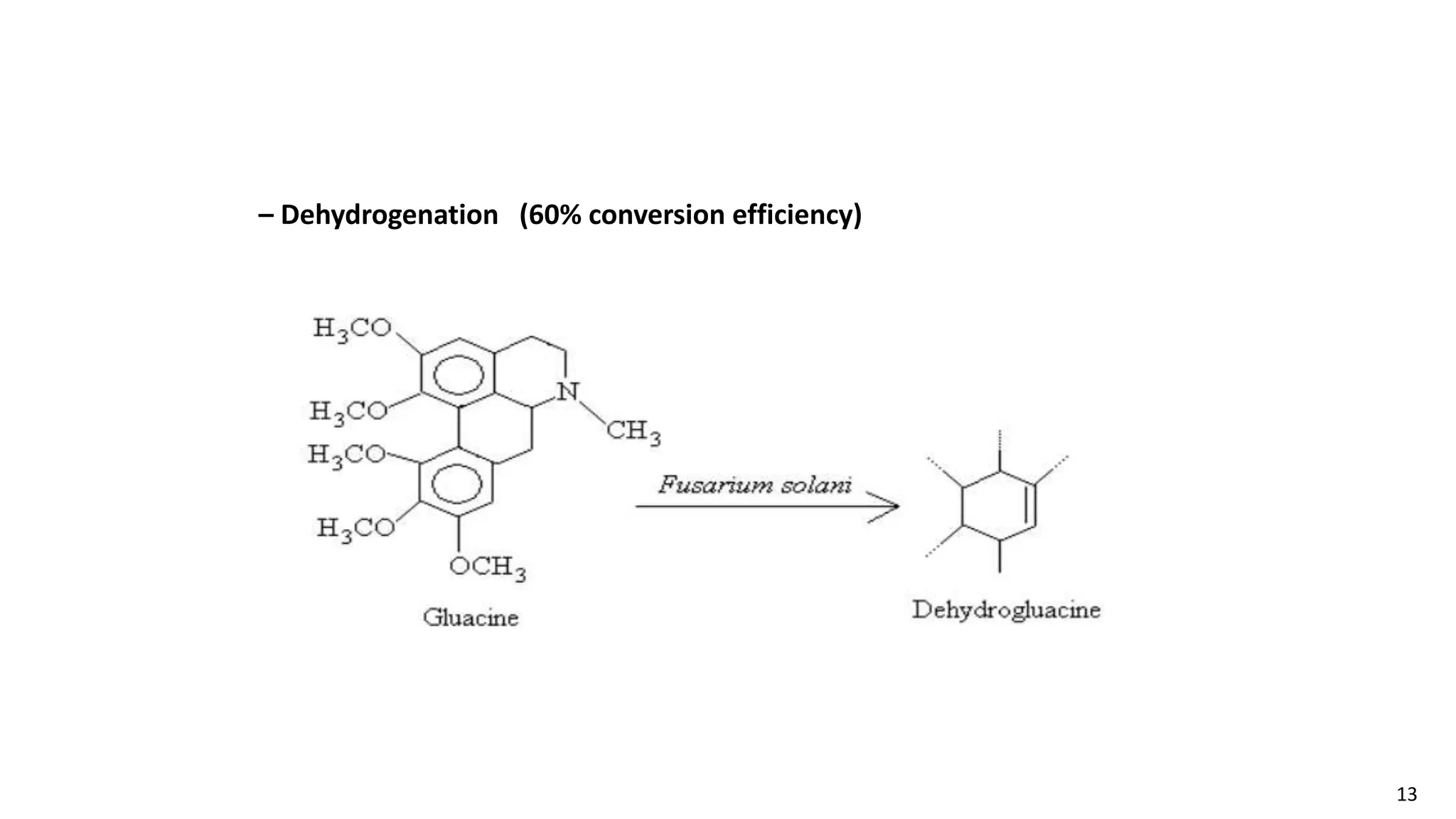
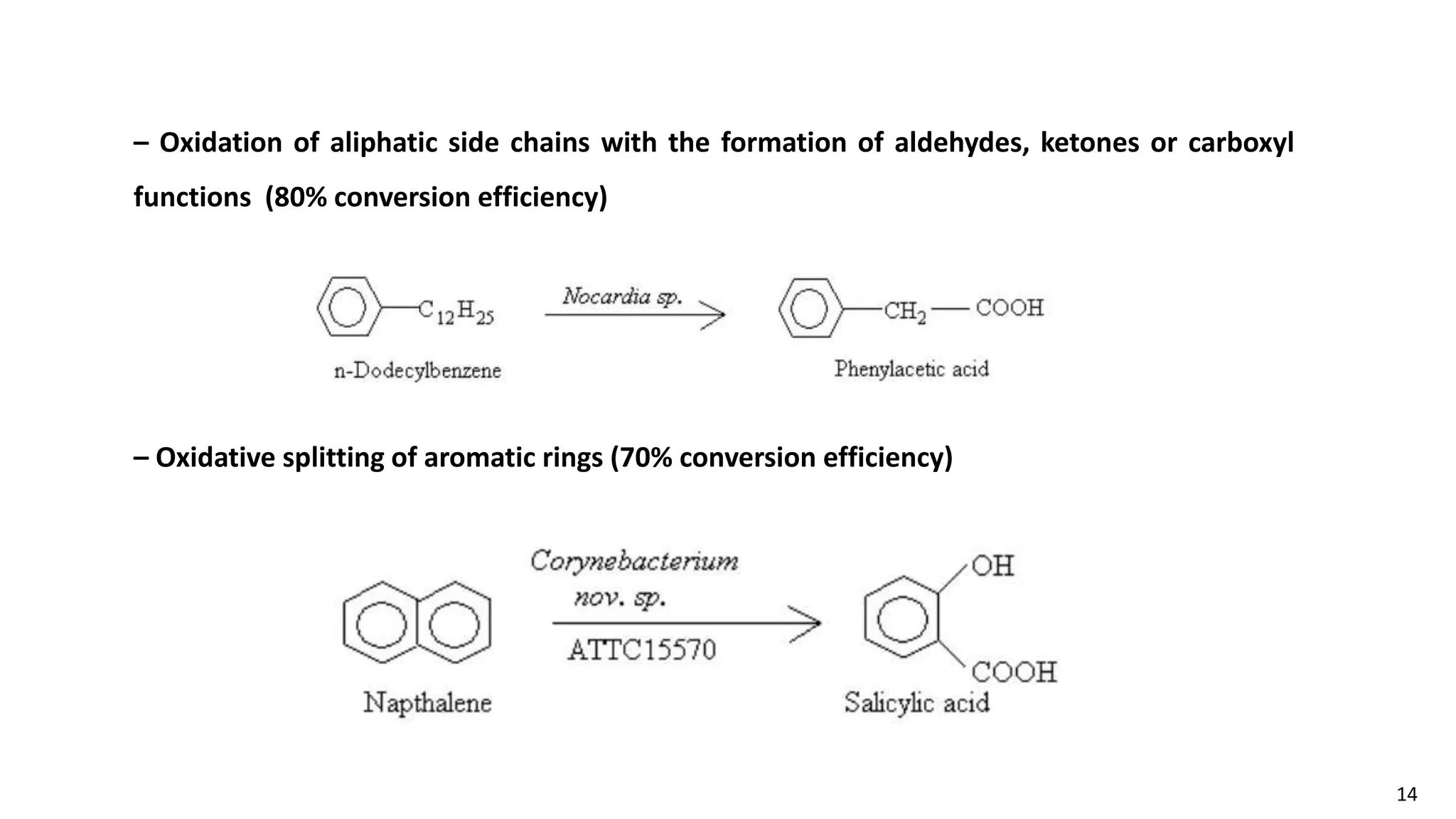
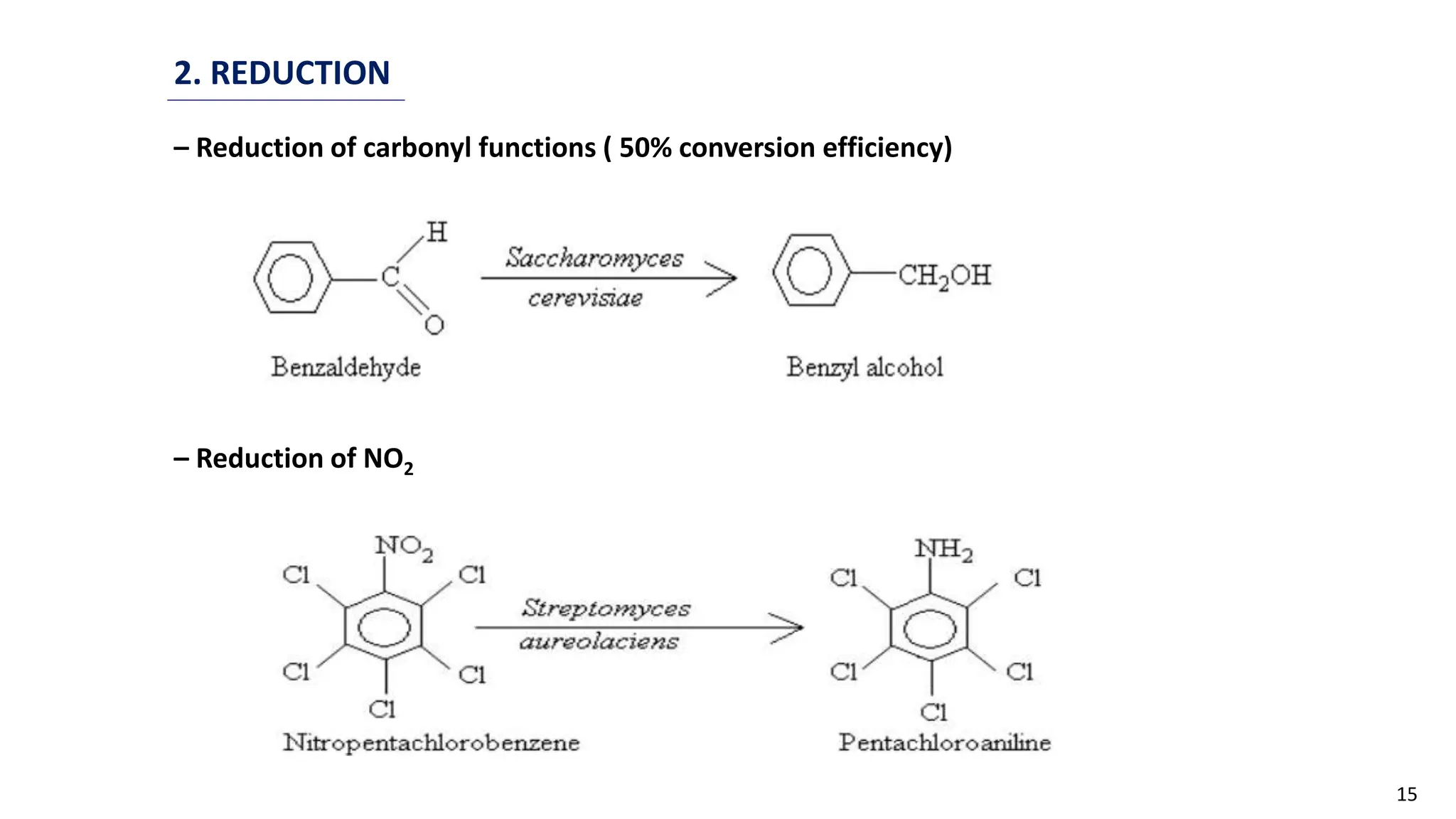
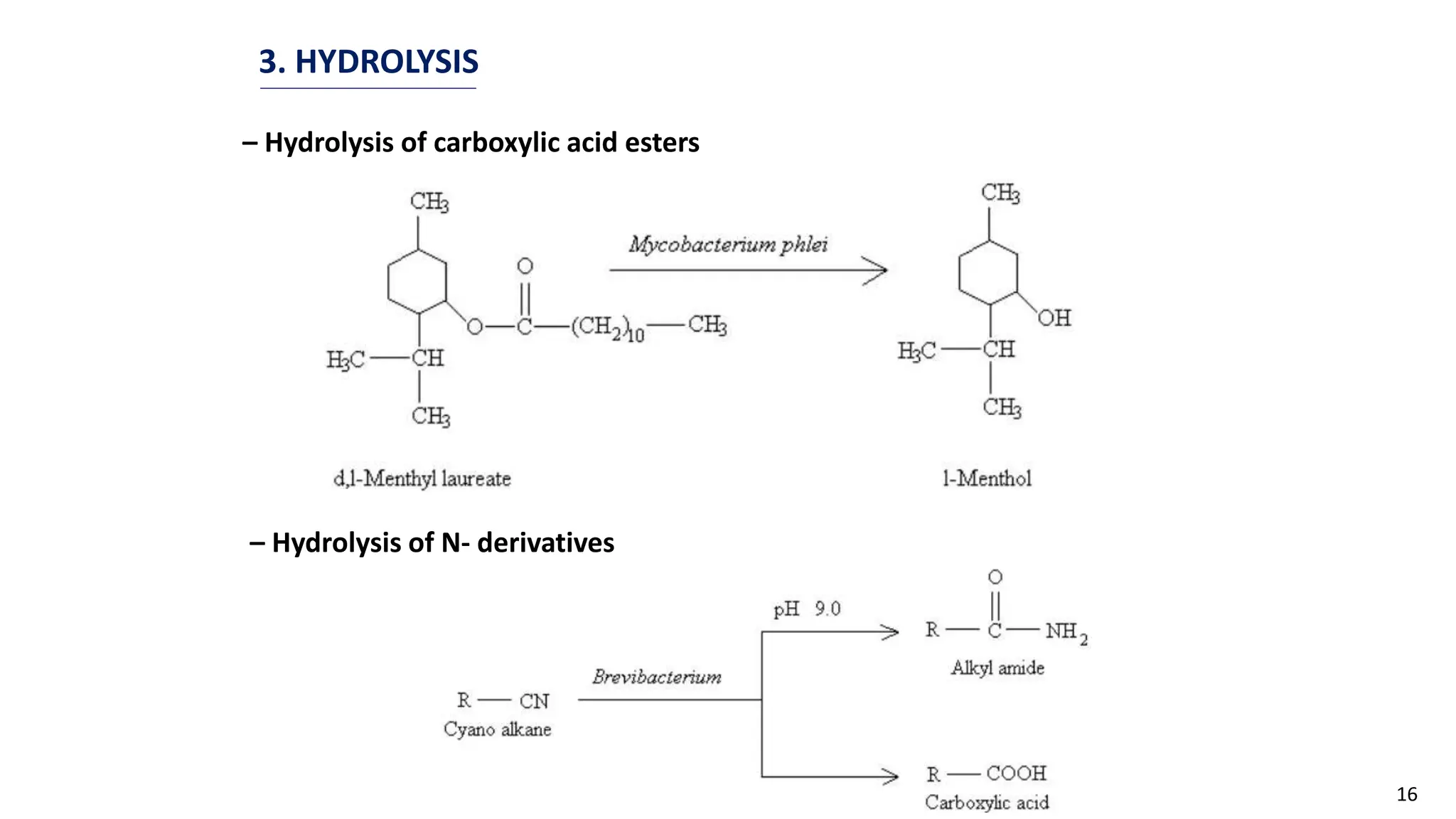
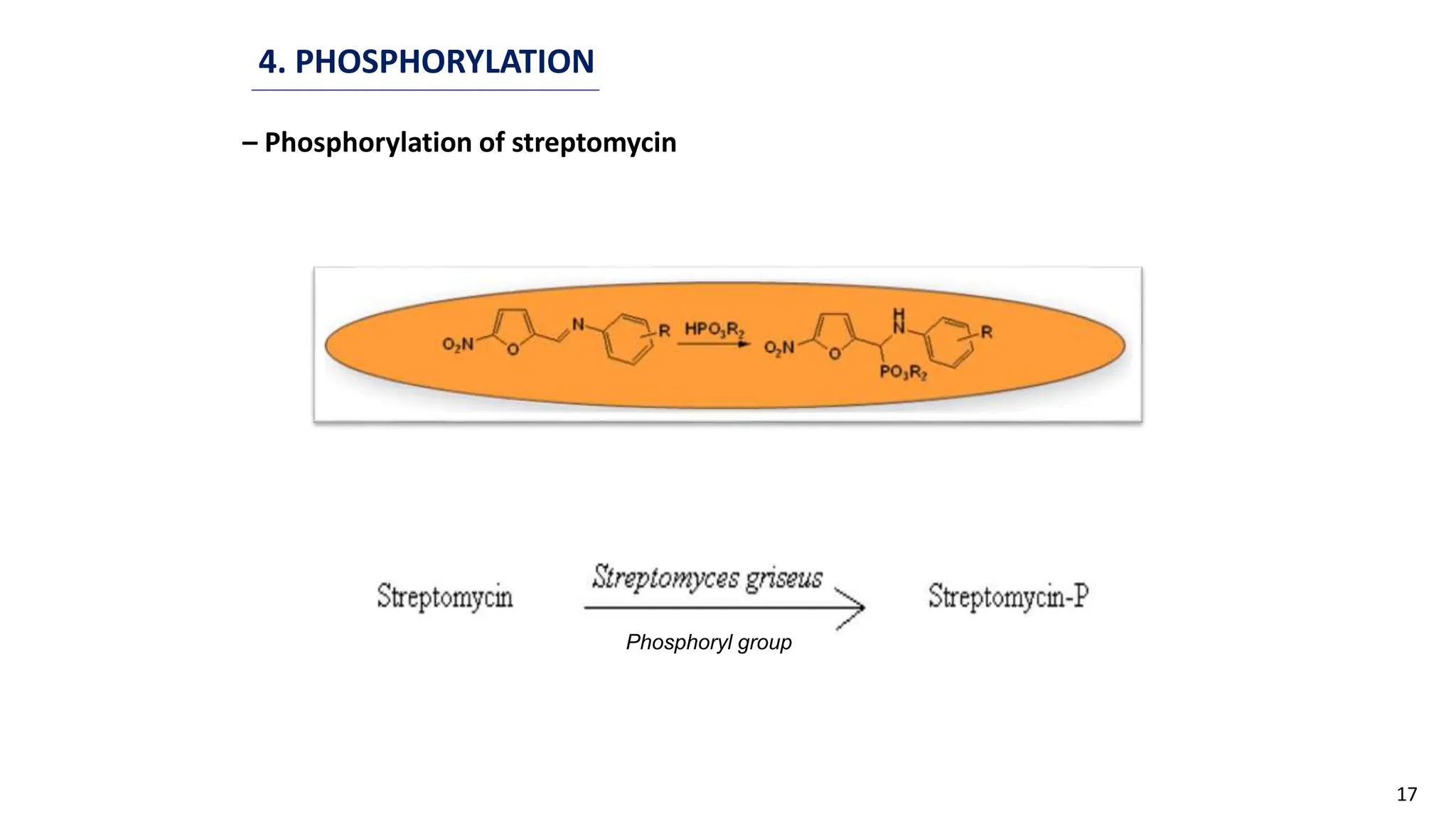
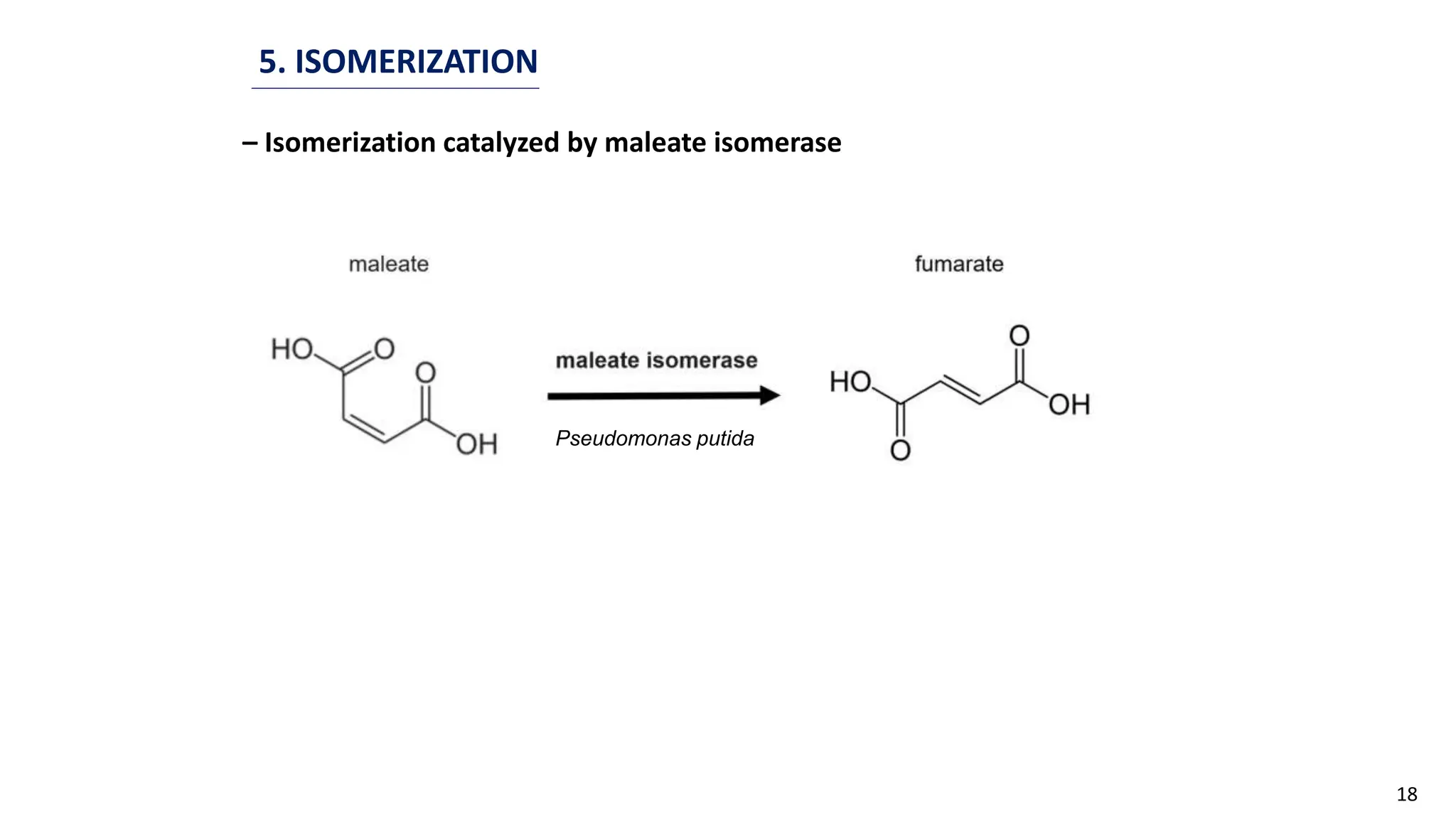
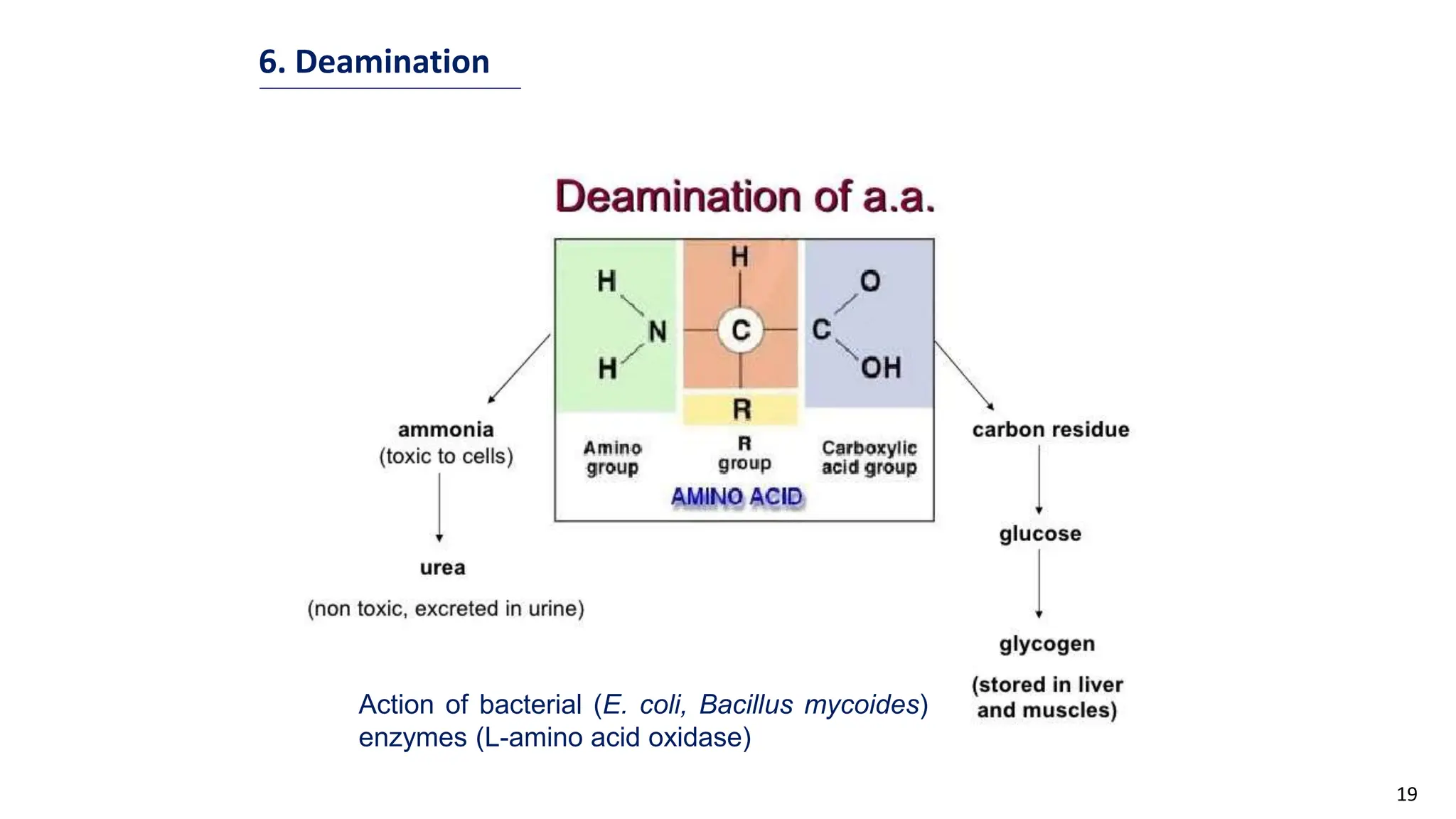
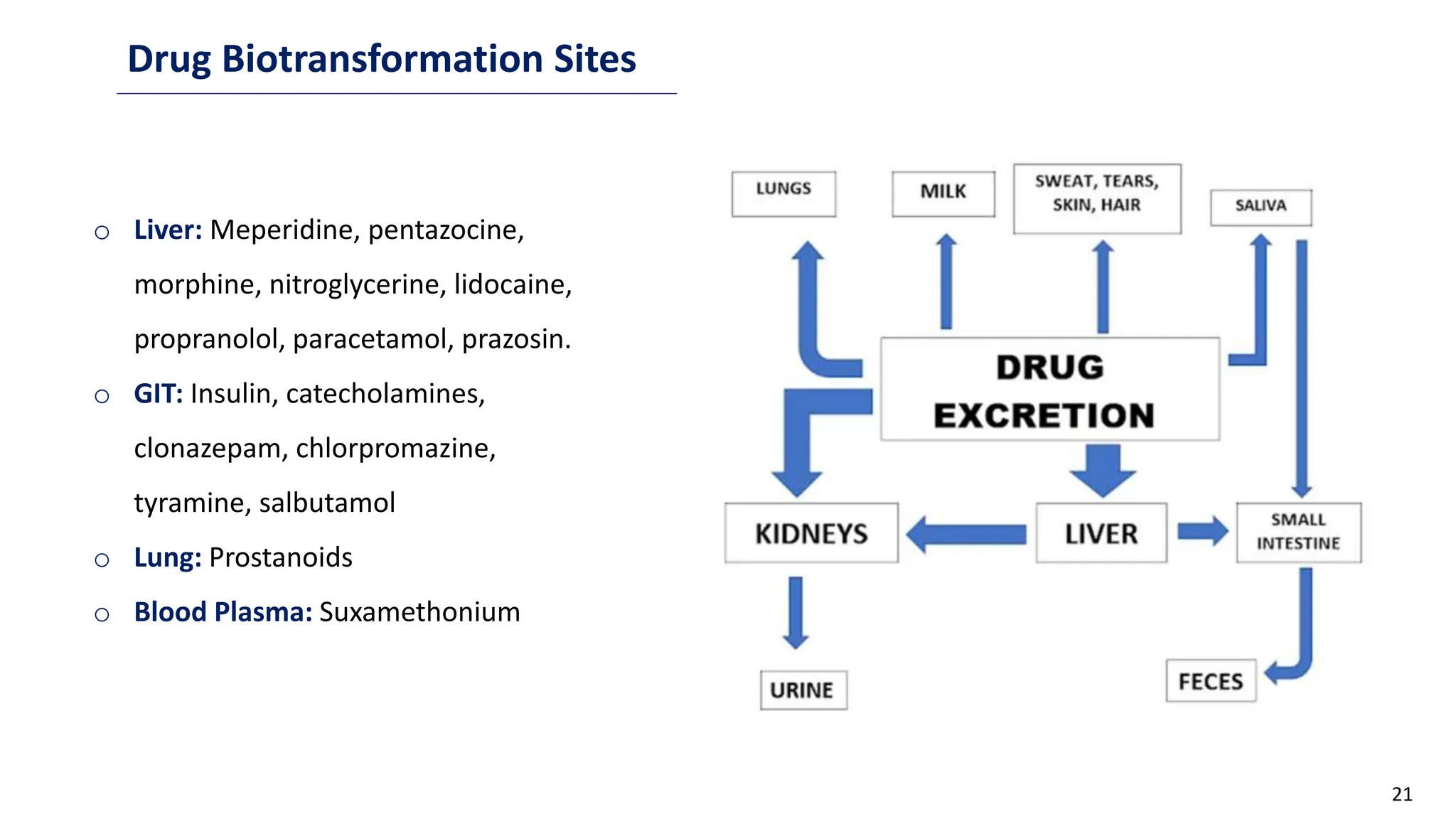
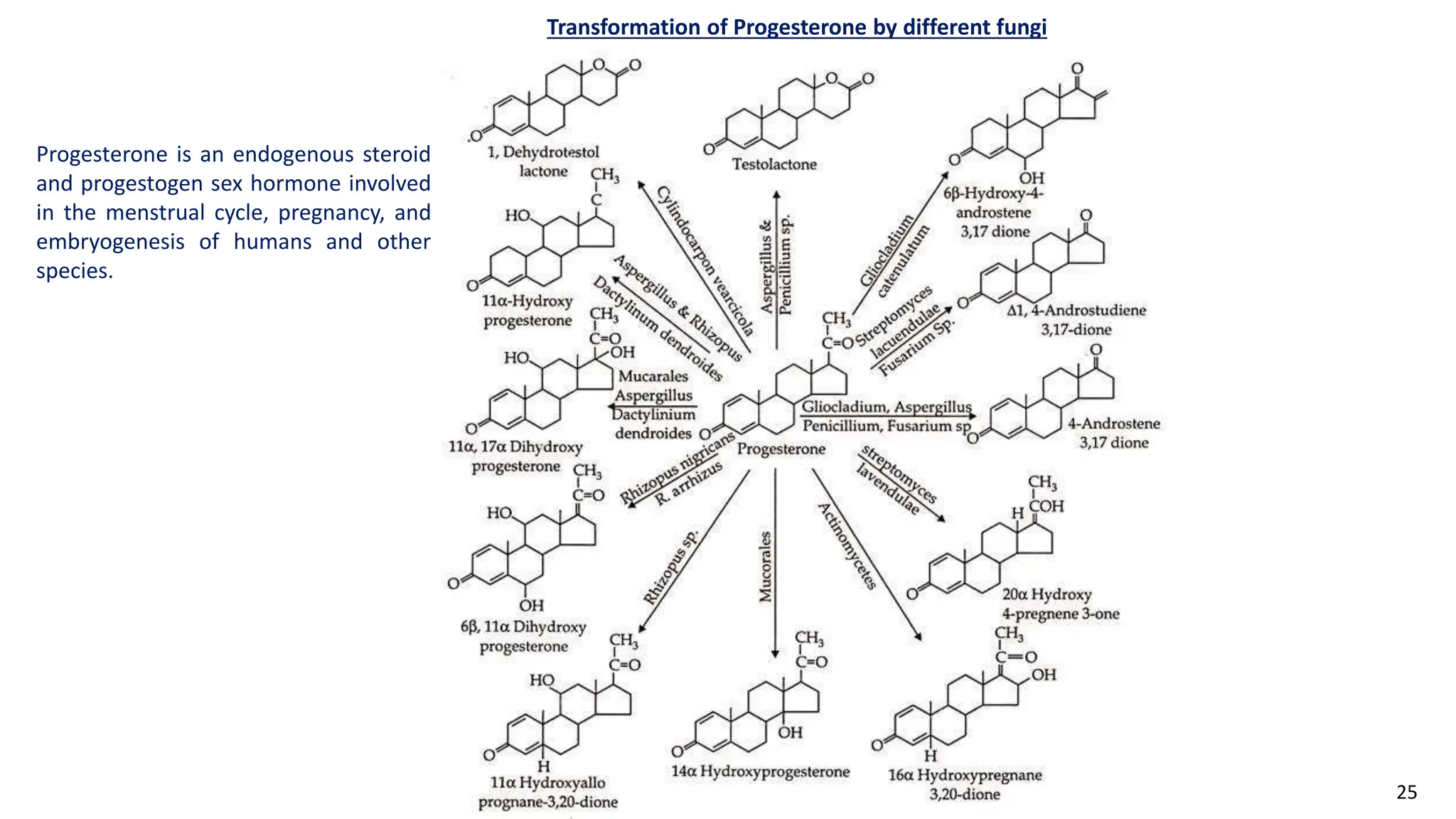
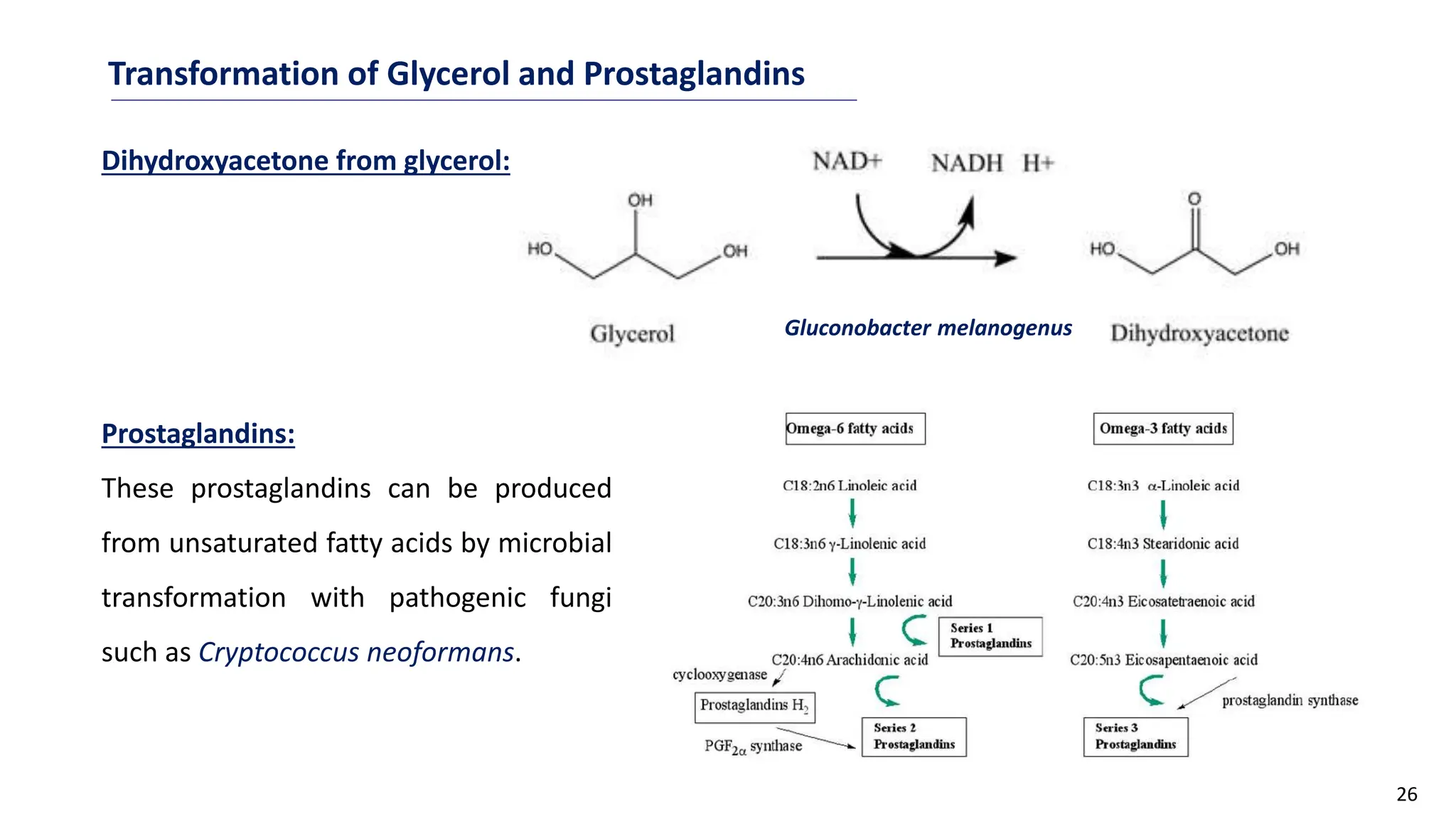
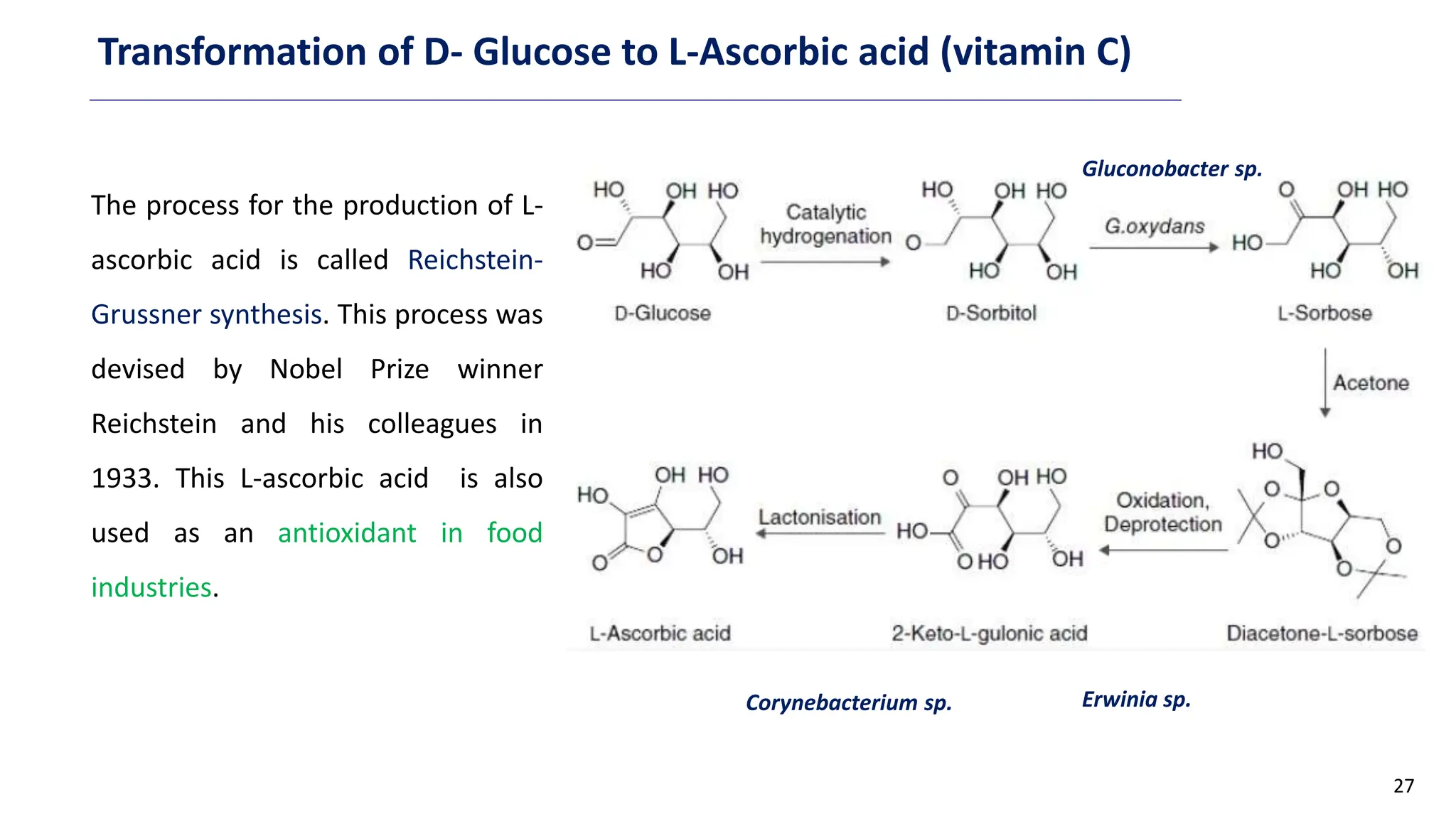
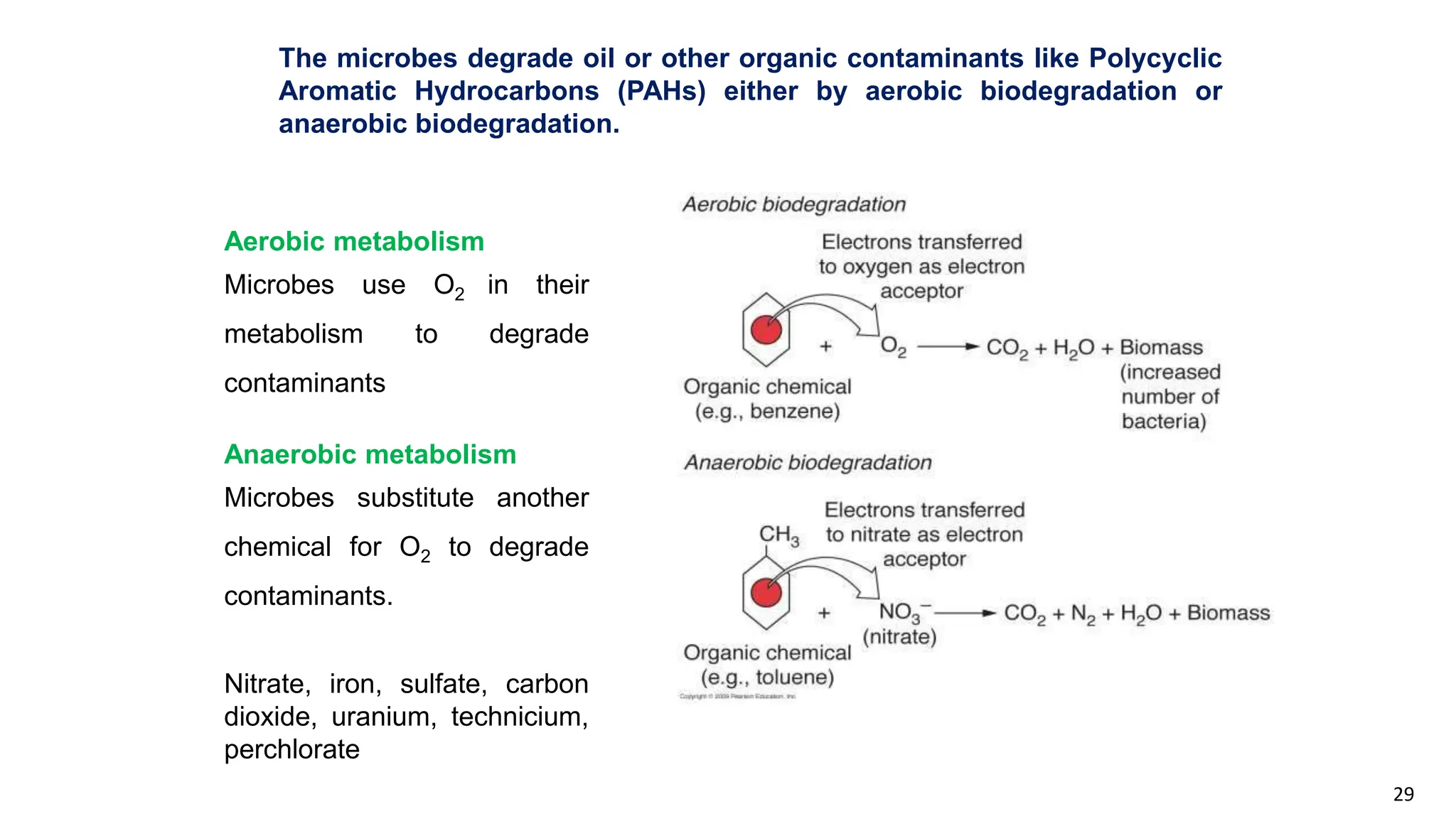
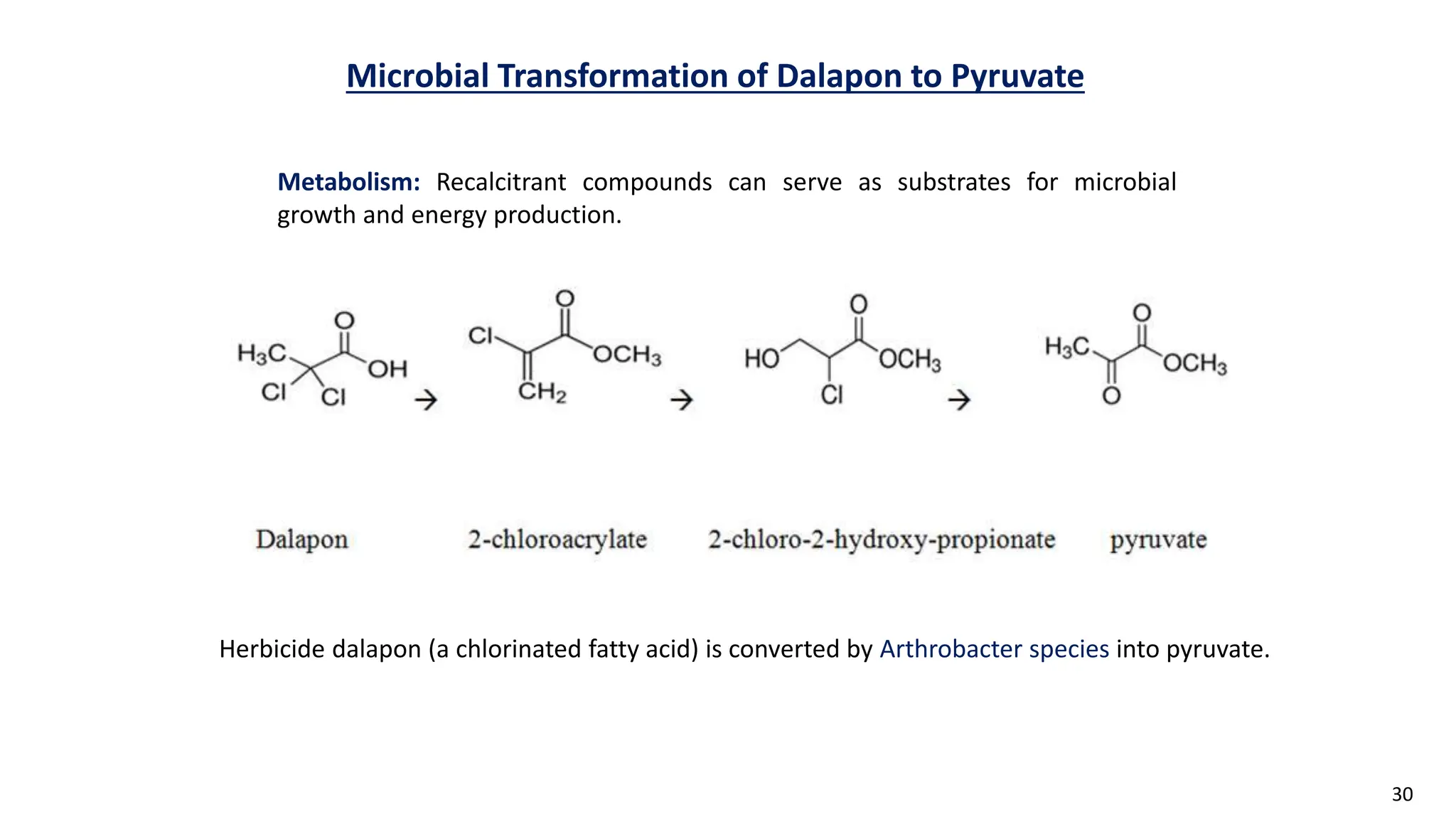
This document discusses microbial transformation of various compounds. It describes how microbes can transform recalcitrant compounds, drugs, steroids, pollutants, dyes and herbicides through various reactions like oxidation, reduction, hydrolysis etc. It compares microbial transformation to chemical synthesis and plant/animal cell transformations, noting advantages like specificity, mild reaction conditions and fewer steps in the microbial process.