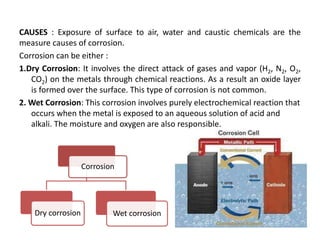
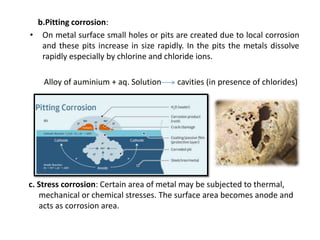
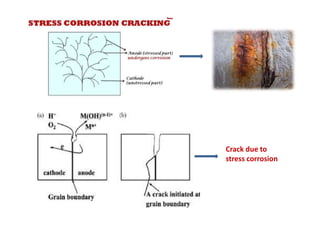
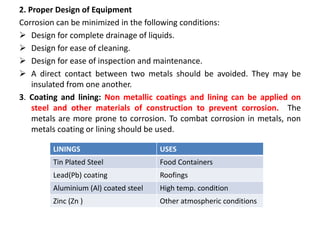
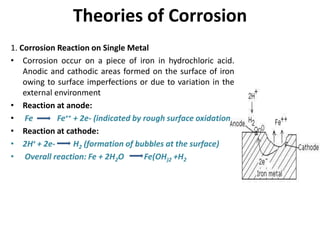
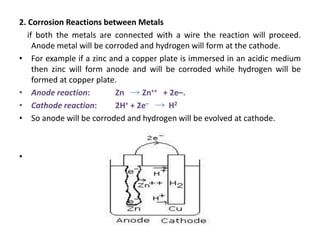
The document discusses factors to consider when selecting materials for constructing pharmaceutical plants. Chemical factors like product contamination and corrosion must be prevented by choosing compatible materials. Physical factors like strength, mass, thermal conductivity, and ability to be sterilized and cleaned easily also influence material selection. Common types of corrosion like general, localized, and galvanic corrosion can be prevented by selecting corrosion-resistant materials, protective coatings, corrosion inhibitors, and cathodic protection. Material choice depends on chemical compatibility, manufacturing requirements, and cost effectiveness.