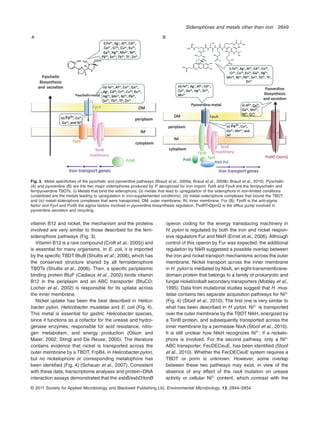
This document discusses new roles for bacterial siderophores in metal transport and tolerance. Siderophores are known to strongly chelate iron, but they can also bind other metals. There is evidence that metals other than iron can induce siderophore production, implicating siderophores in homeostasis of multiple metals. Siderophores form stable complexes with various metals, including some heavy metals, and may contribute to bacterial tolerance against metal toxicity. The ability of siderophores to transport both essential and non-essential metals suggests they influence metal migration in the environment.