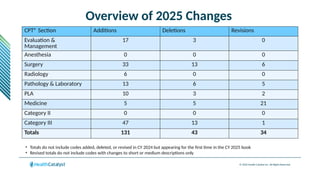
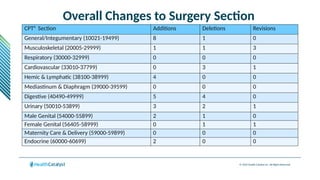
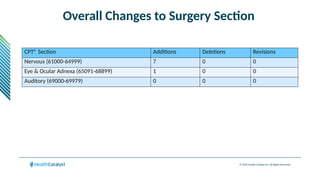
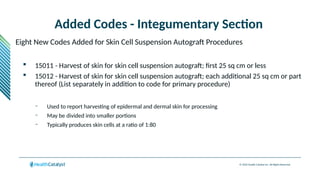
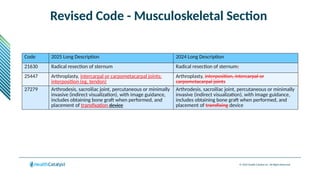
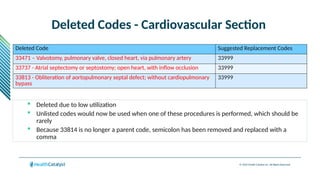
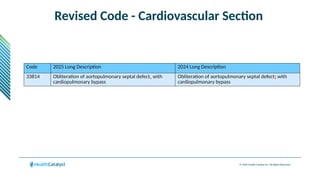
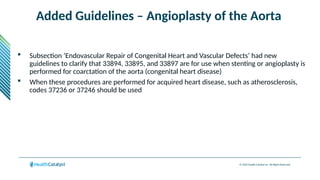
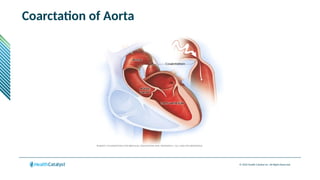
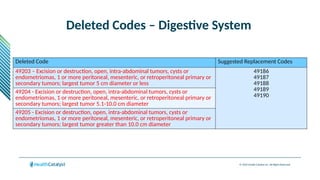
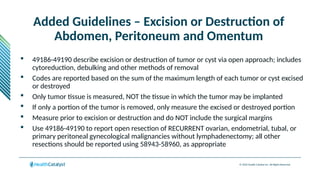
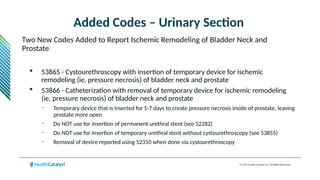
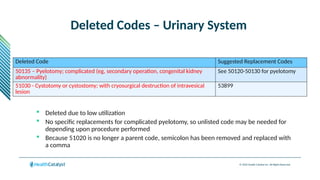
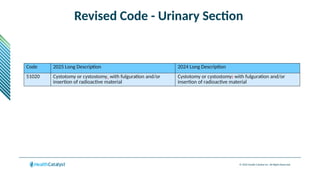
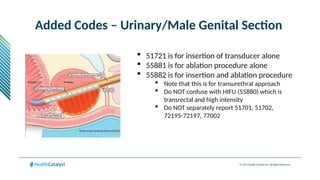
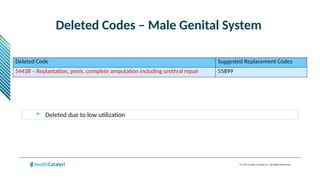
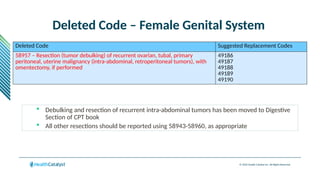
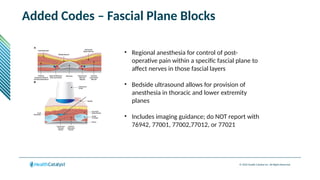
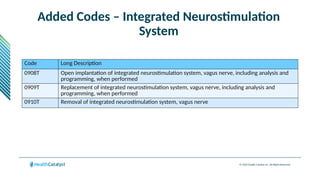
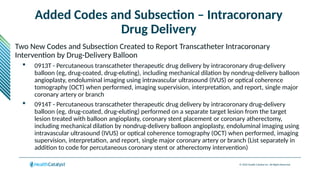
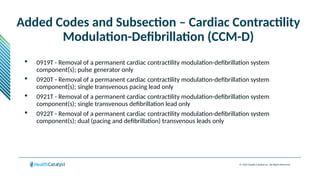
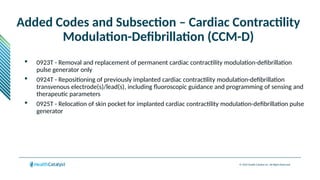
The document provides an overview of the 2025 updates to CPT codes, highlighting a total of 131 code changes, including 43 additions and 34 deletions across various sections such as evaluation and management, surgery, and radiology. Specific sections have detailed changes, such as the addition of new codes for skin cell suspension autografts and guidelines for reporting procedures. It also includes information about deleted codes and the rationale behind some of the changes, emphasizing the importance of reviewing coding policies and regulations.