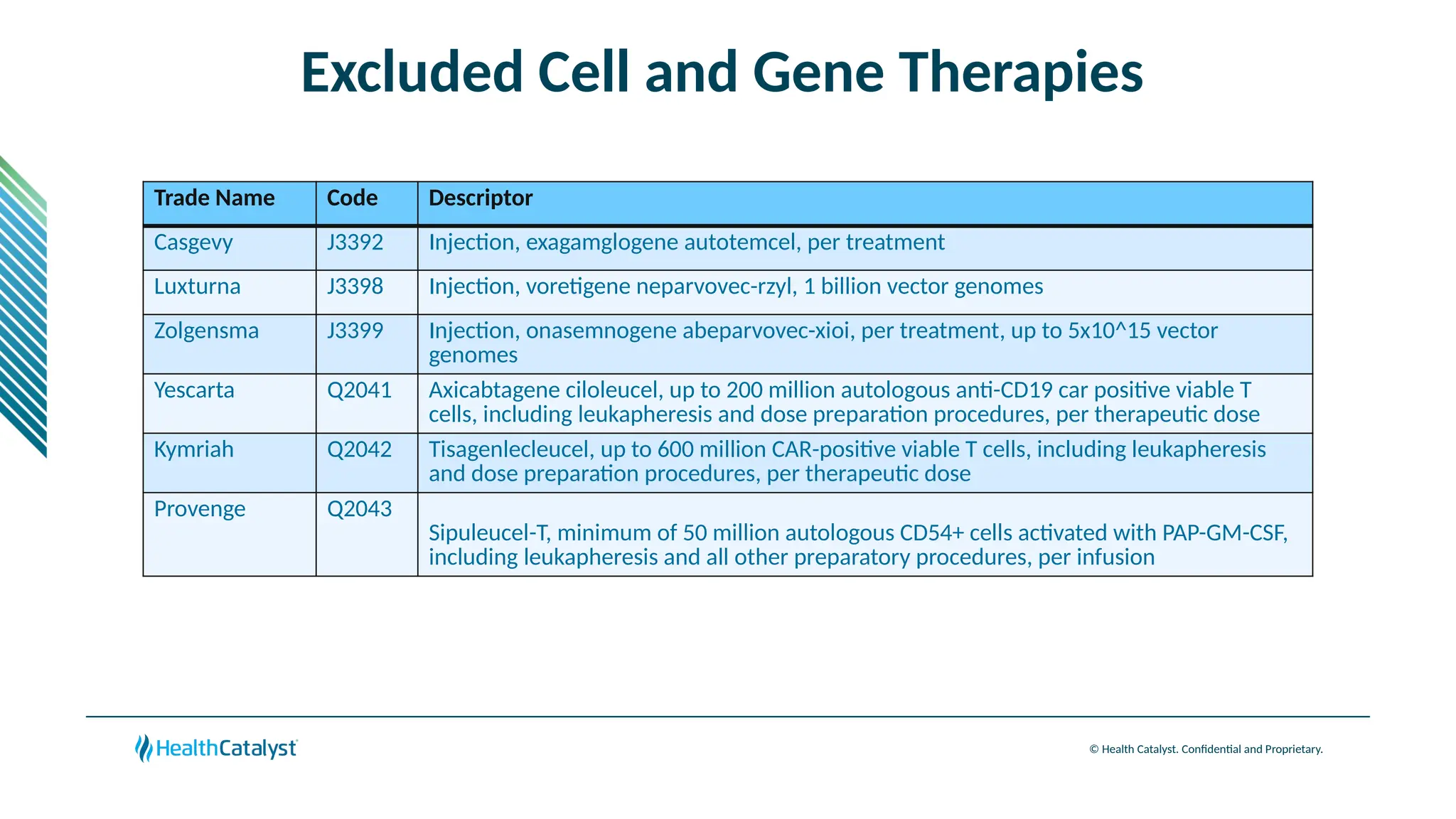
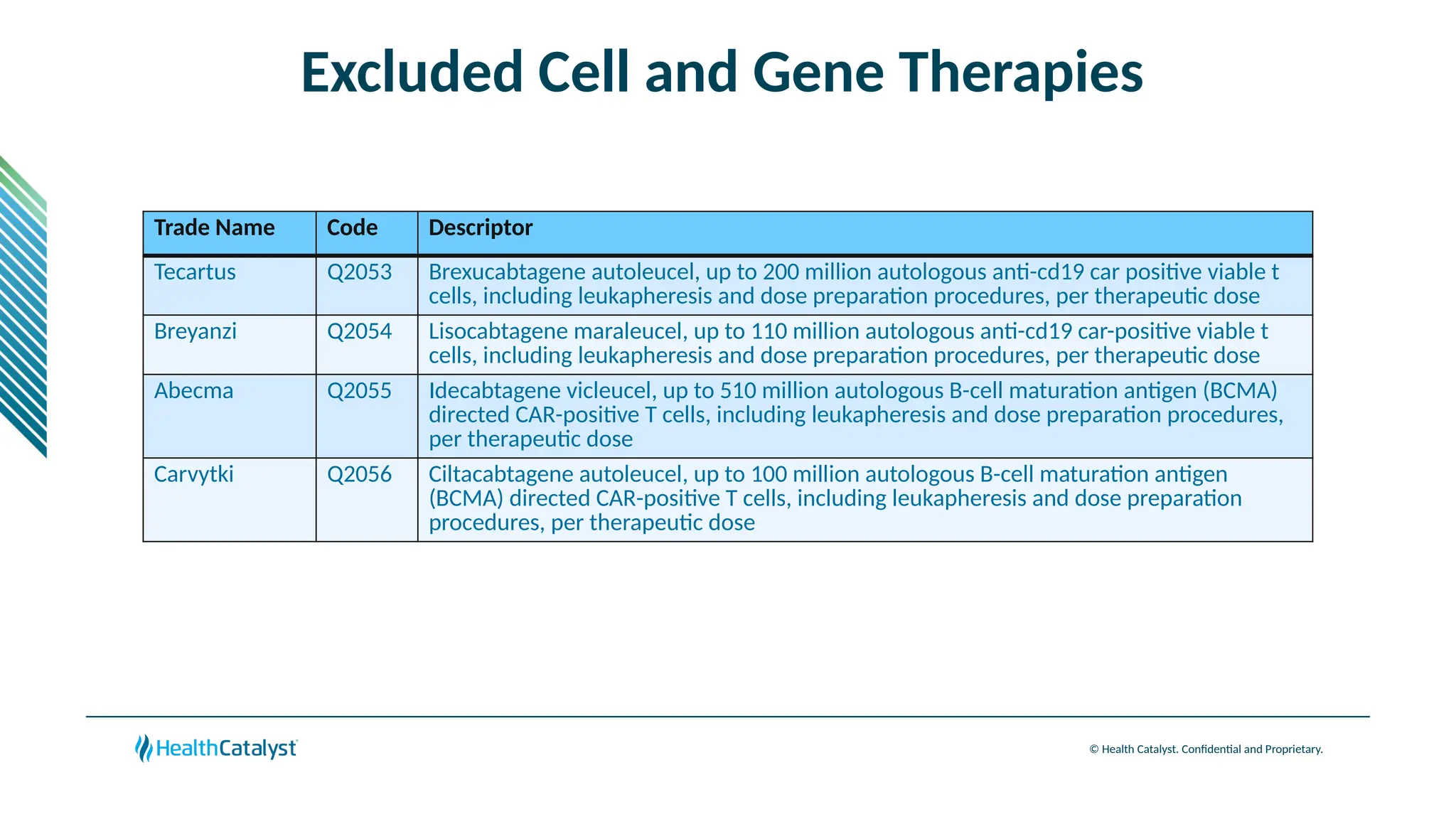
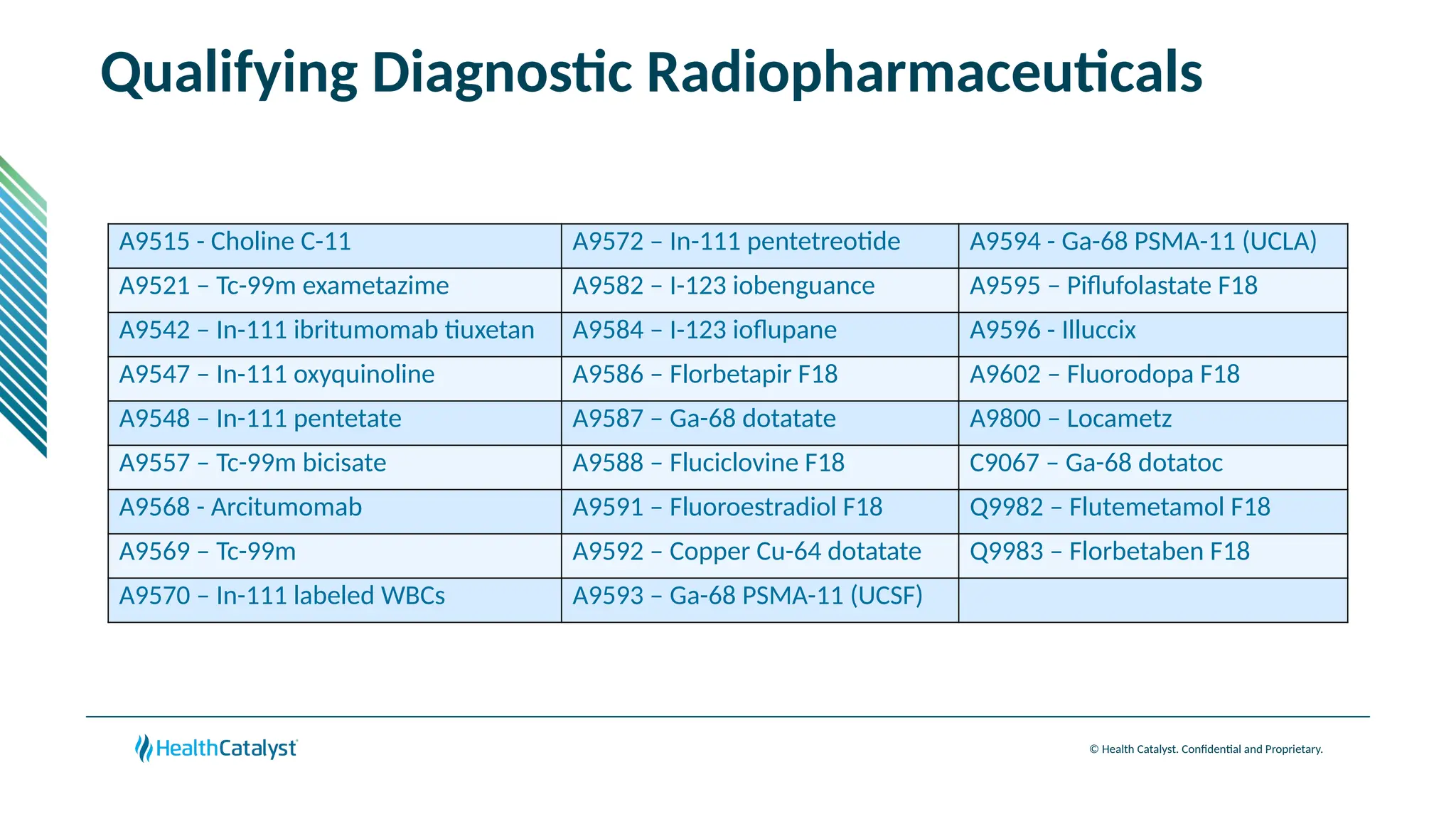
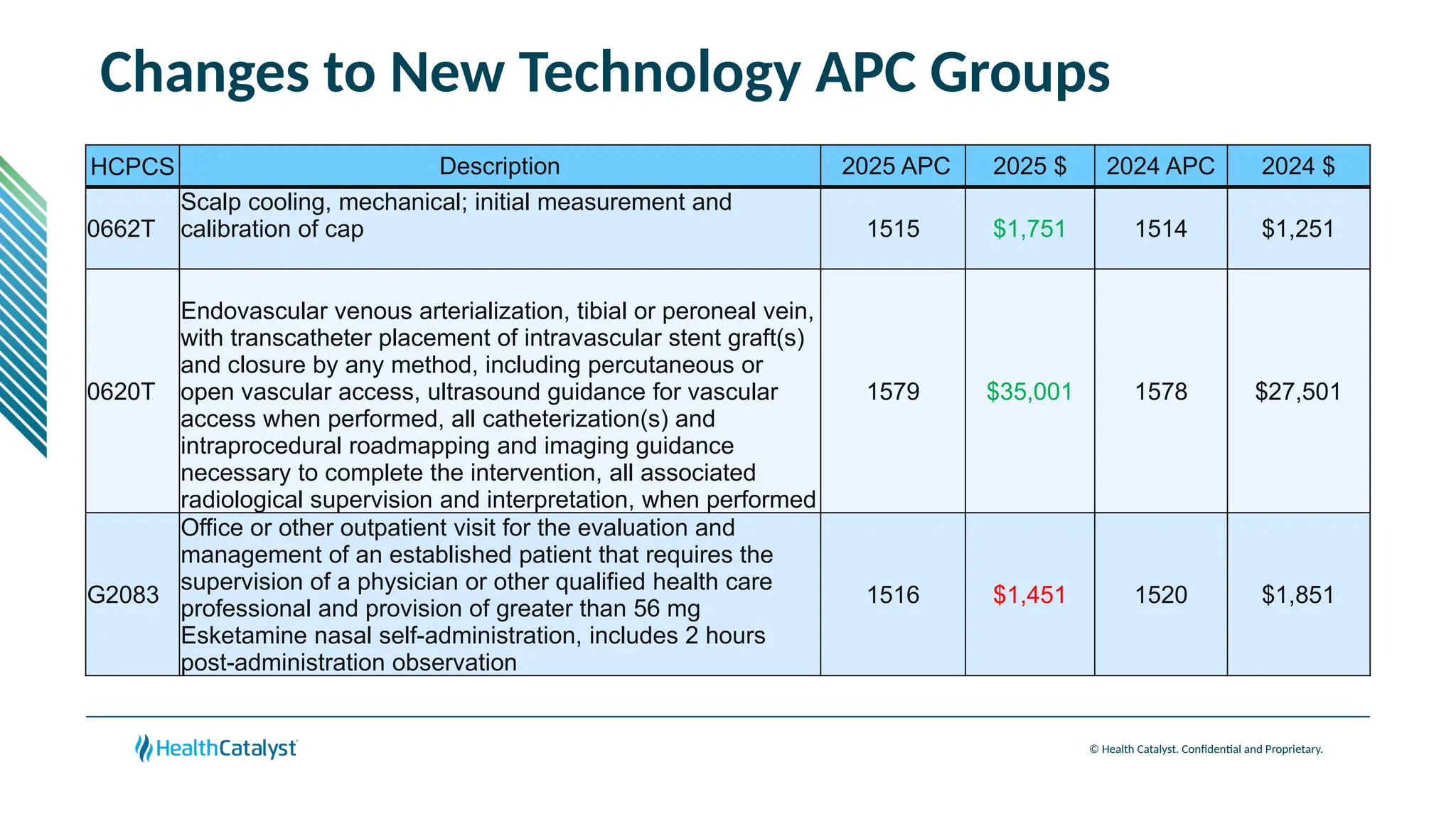
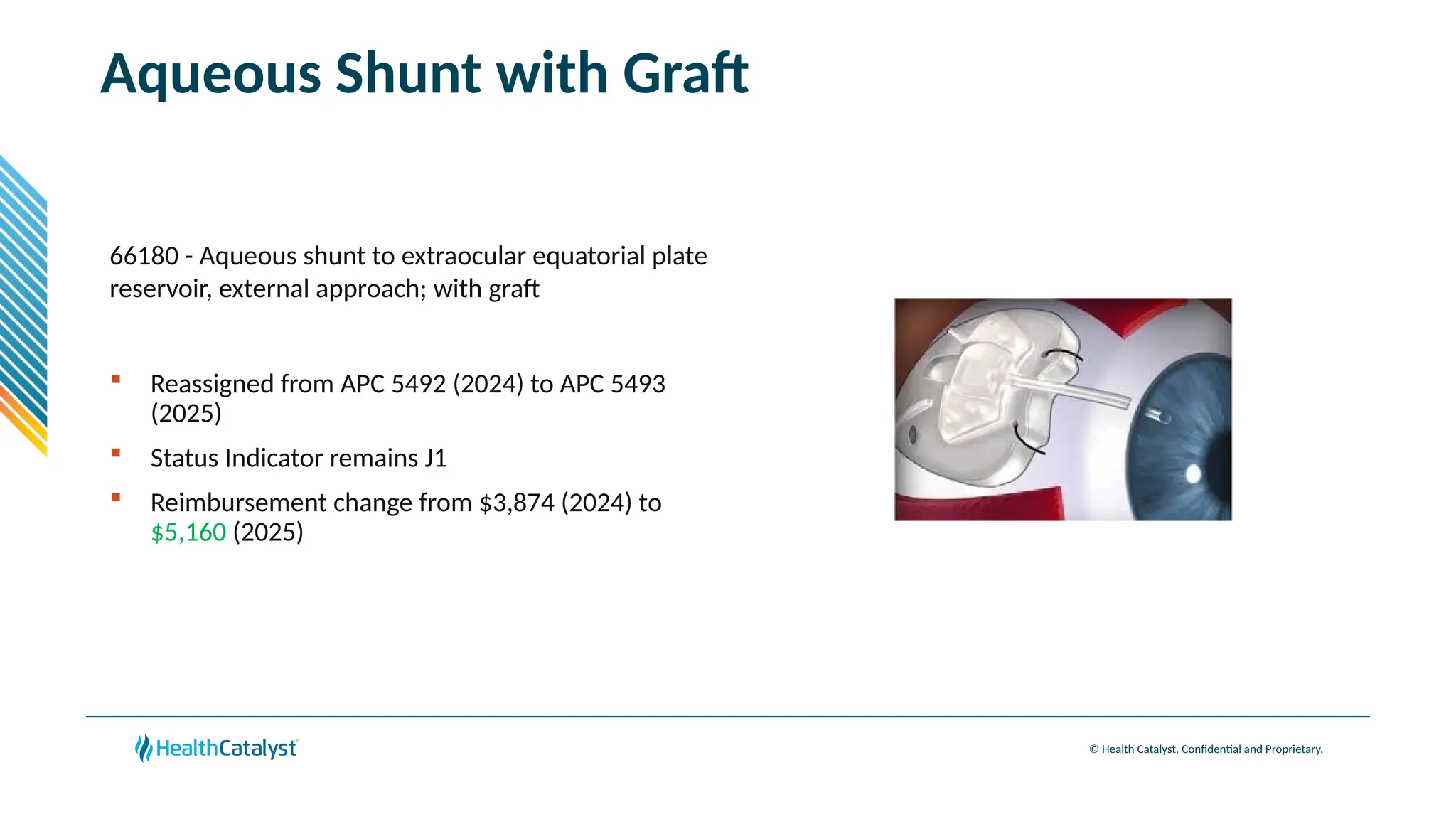
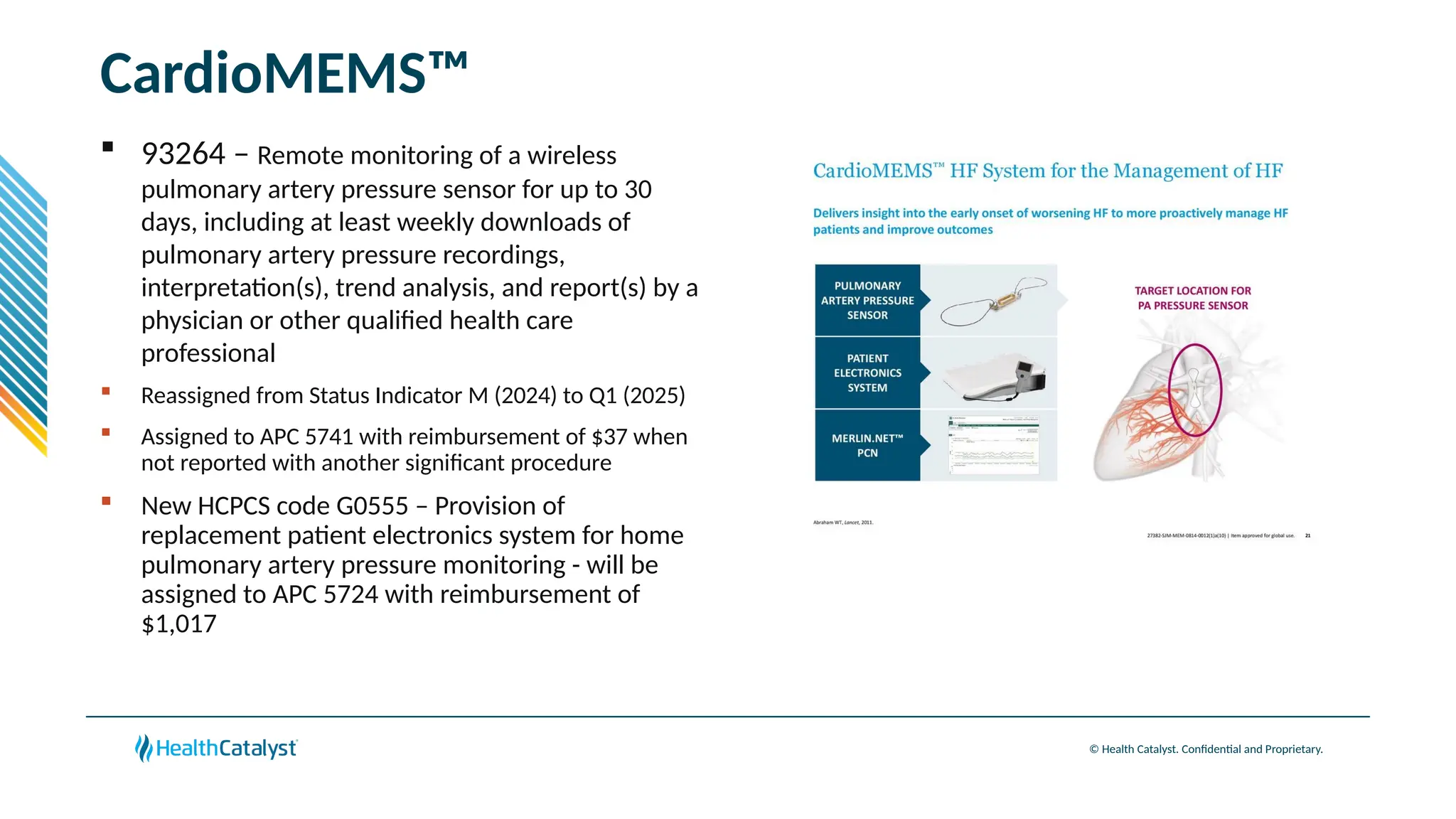
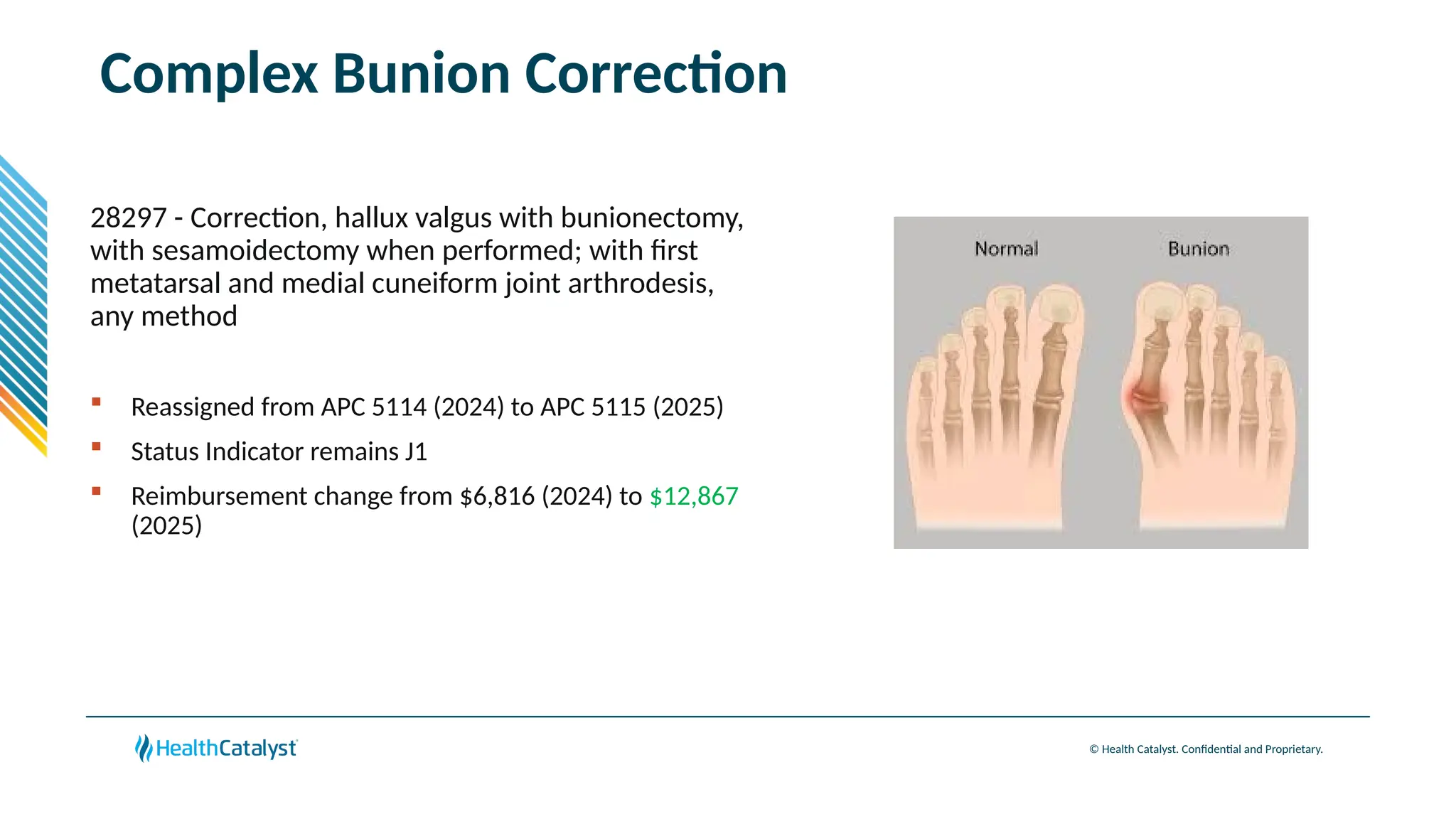
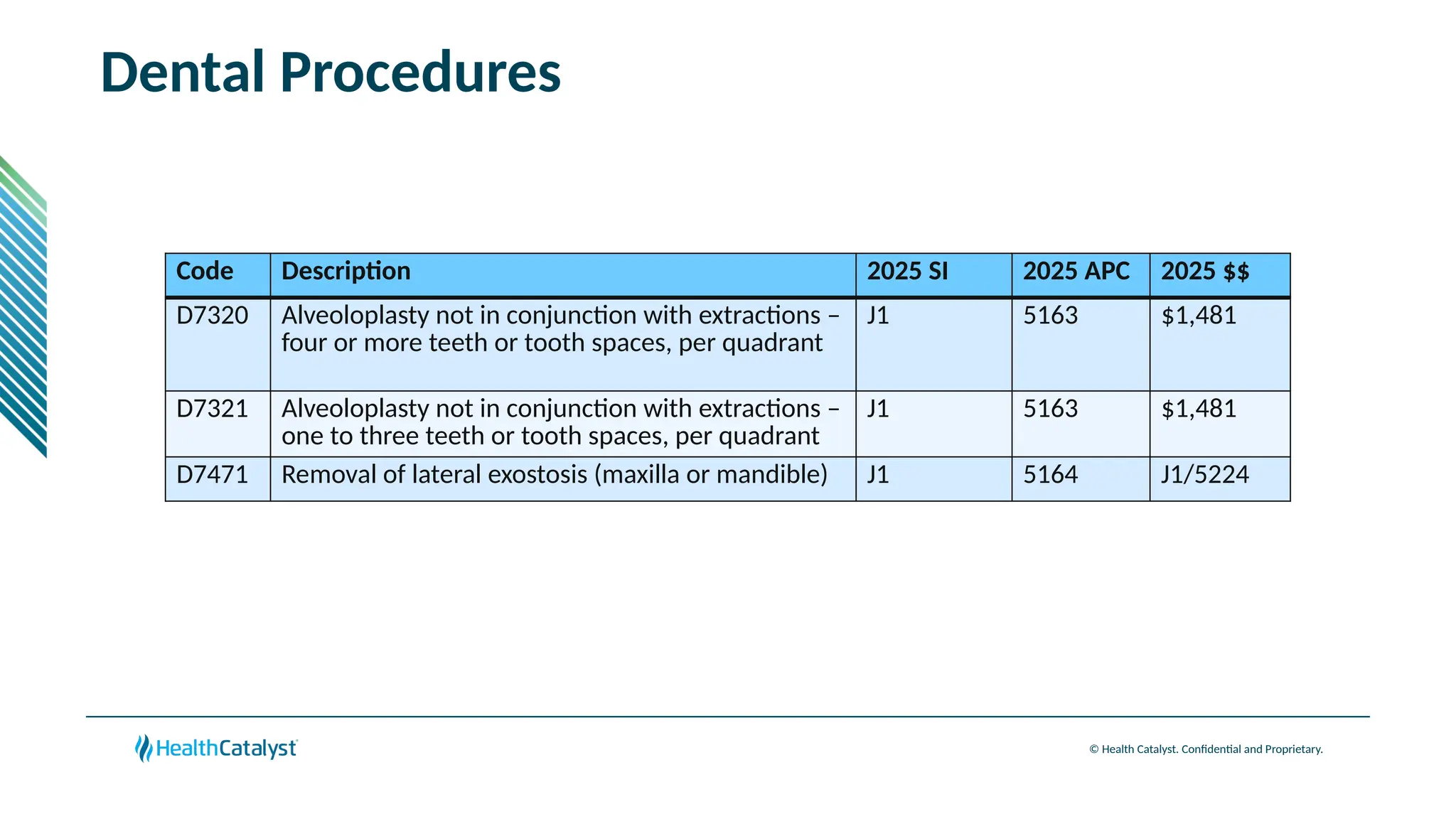
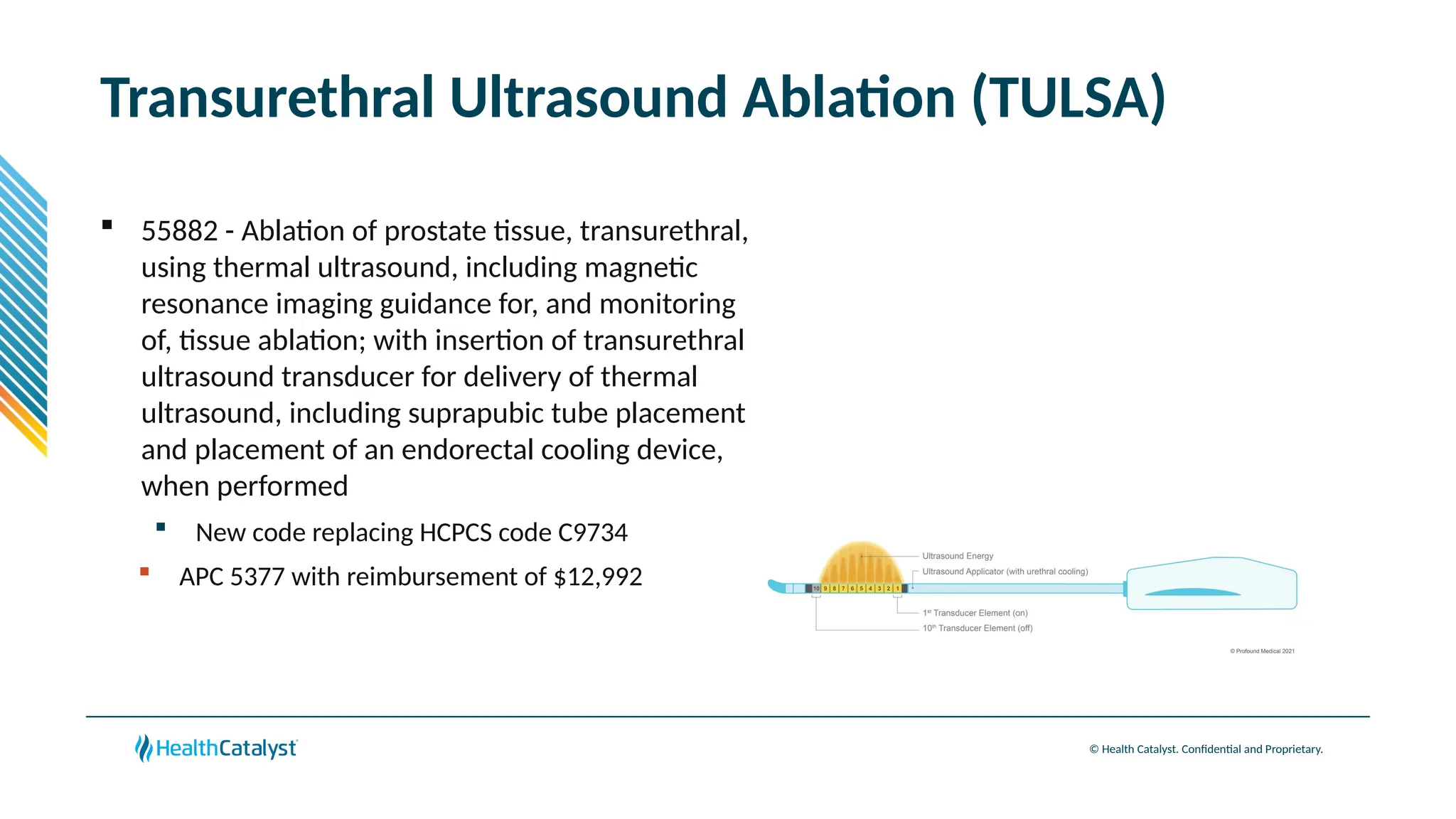
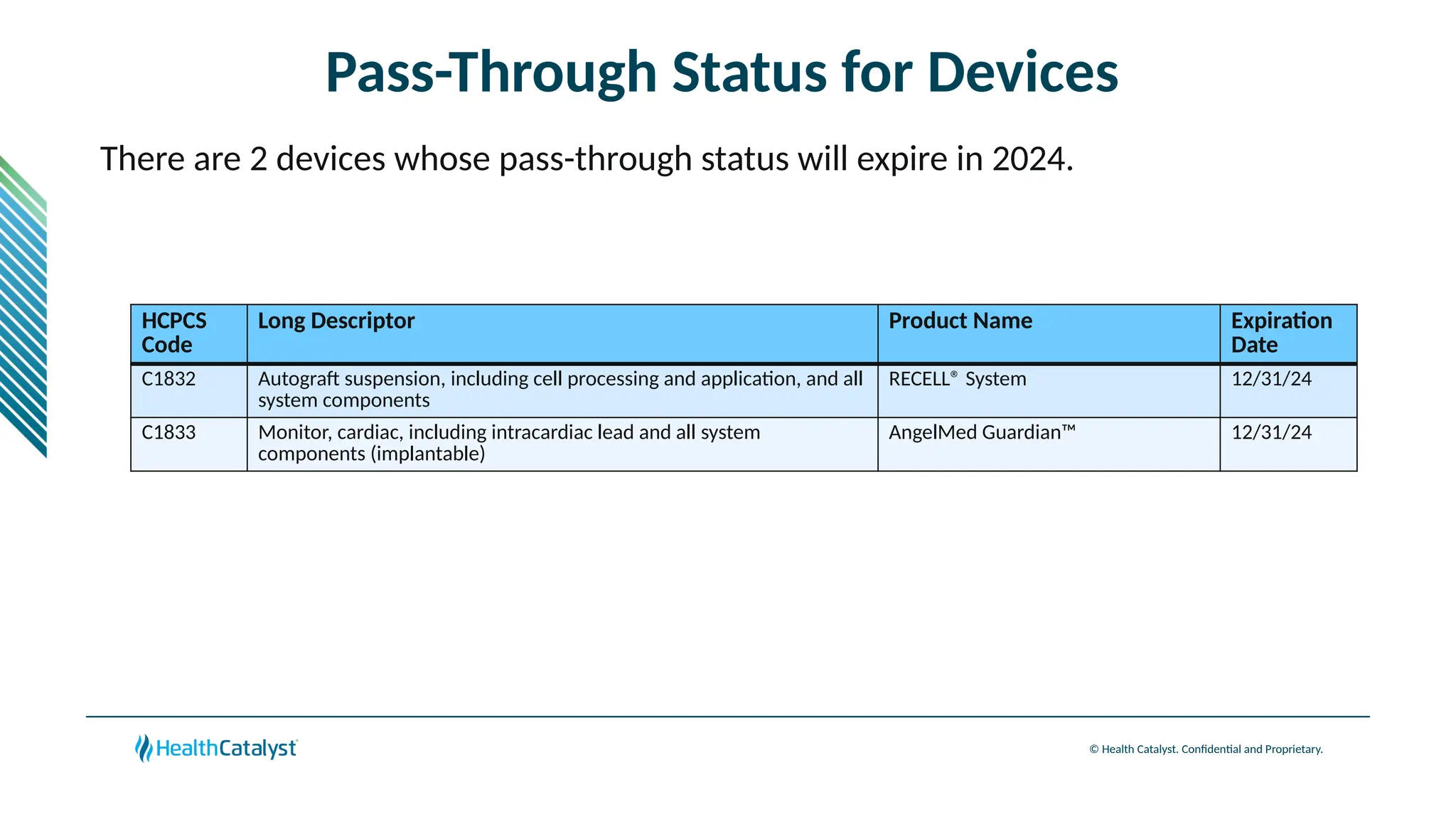
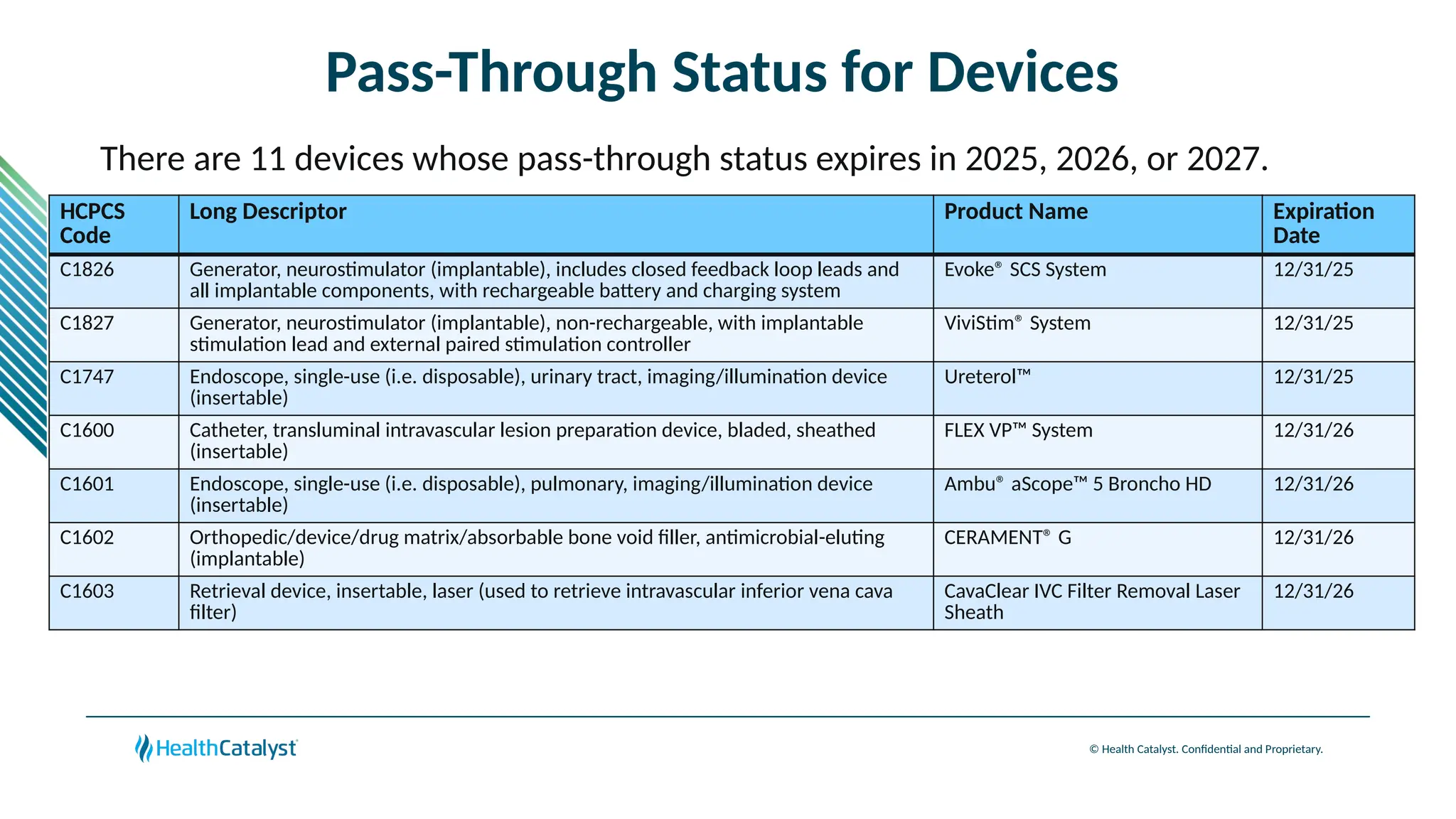
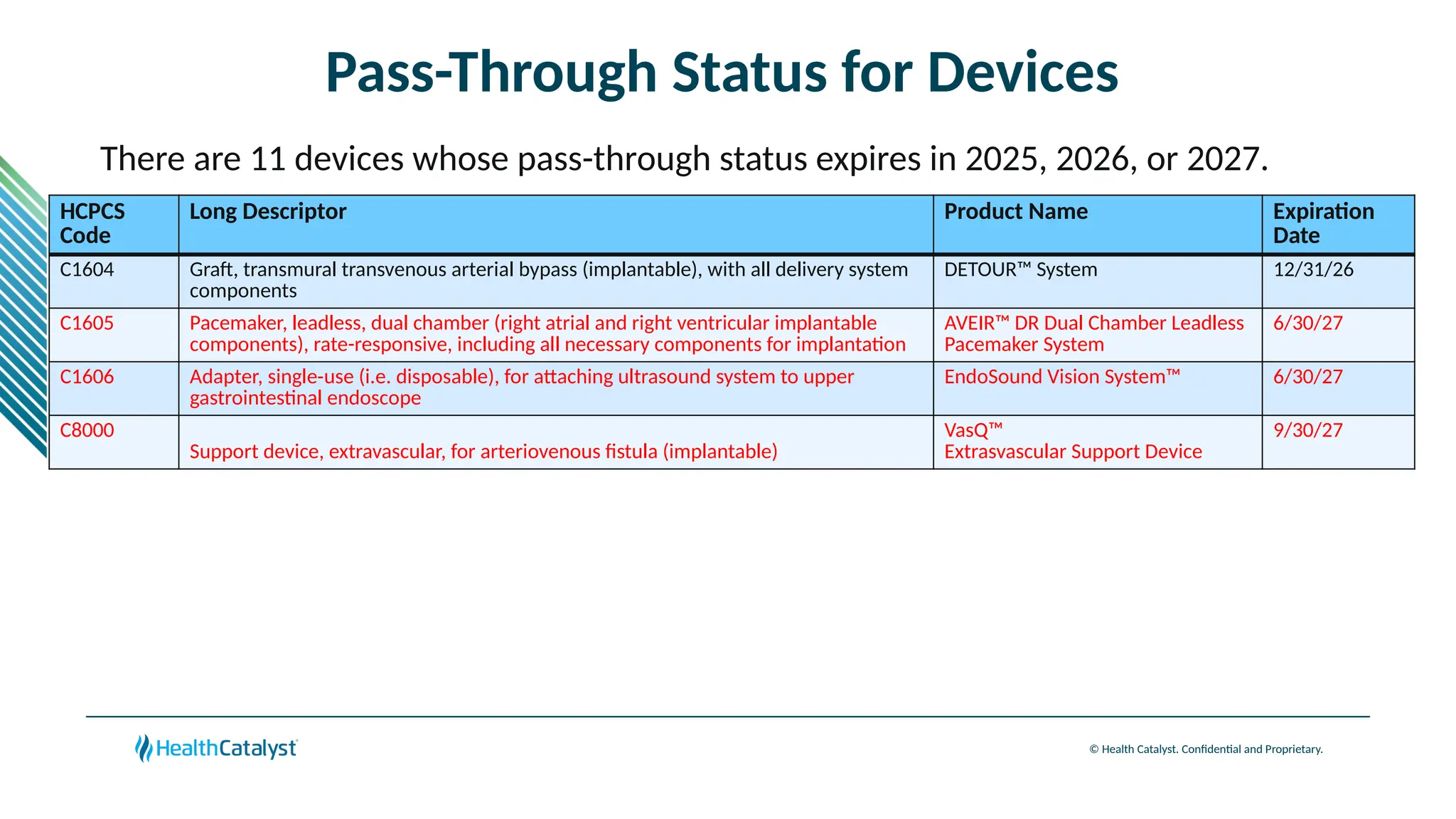
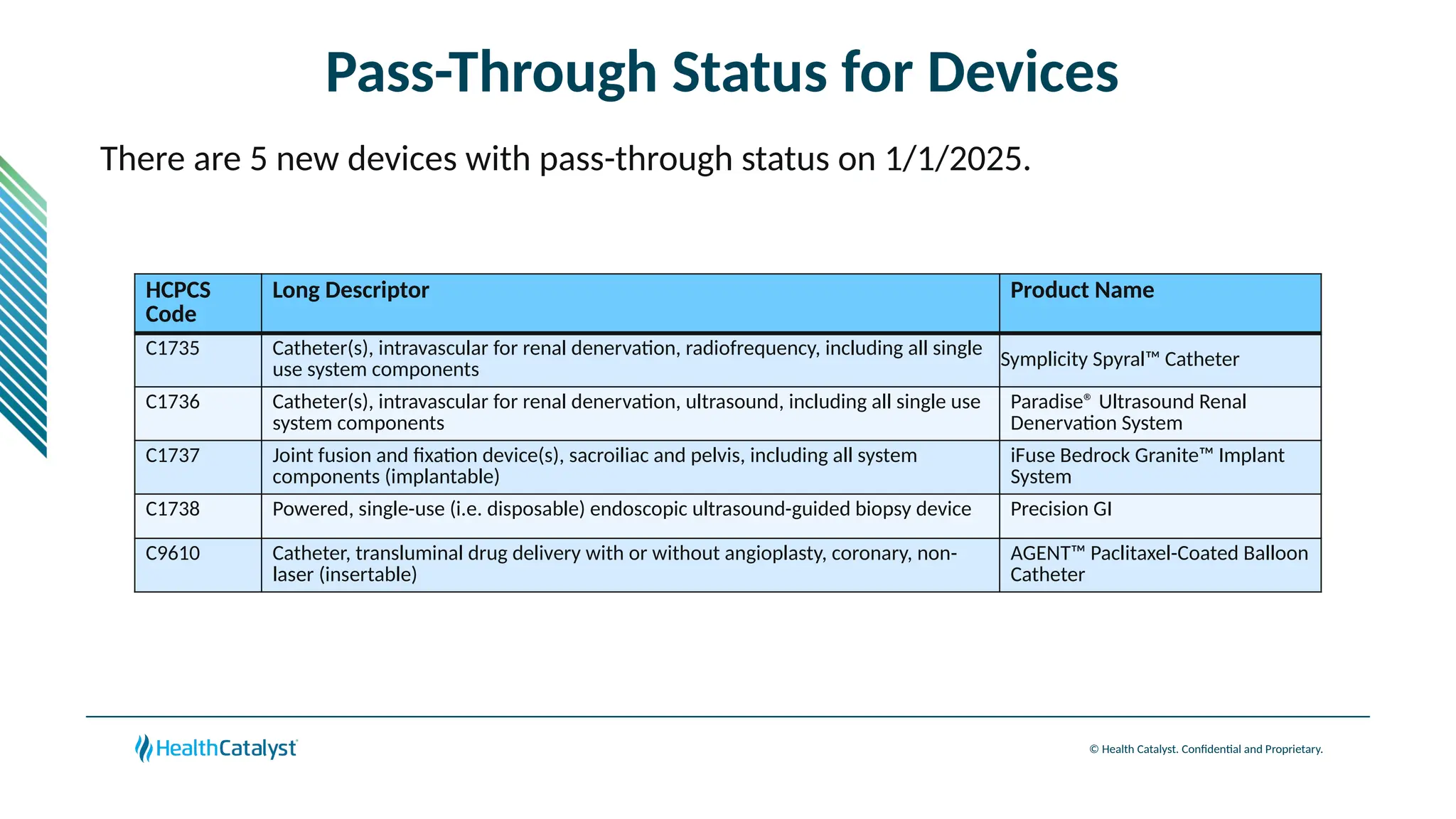
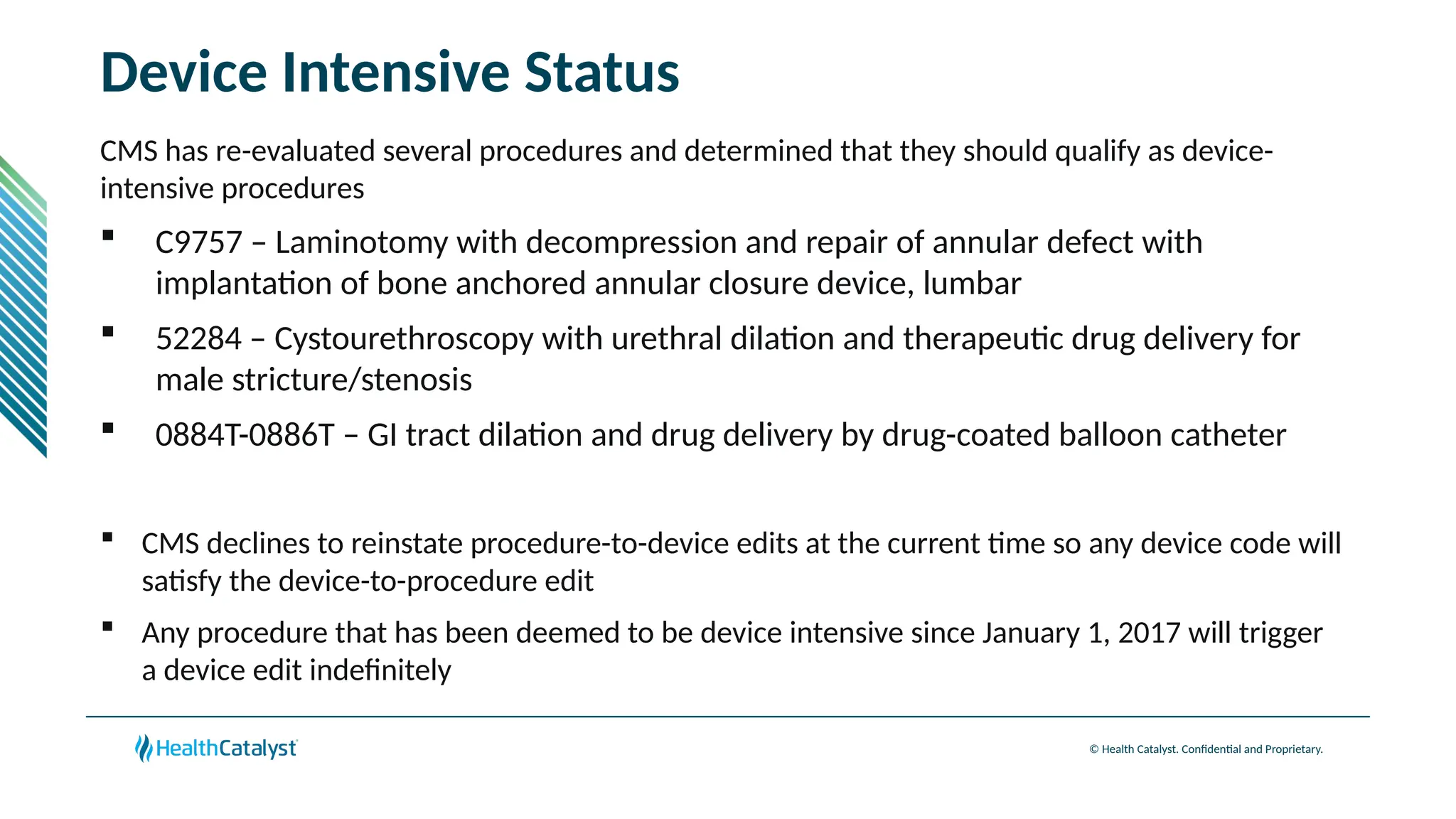
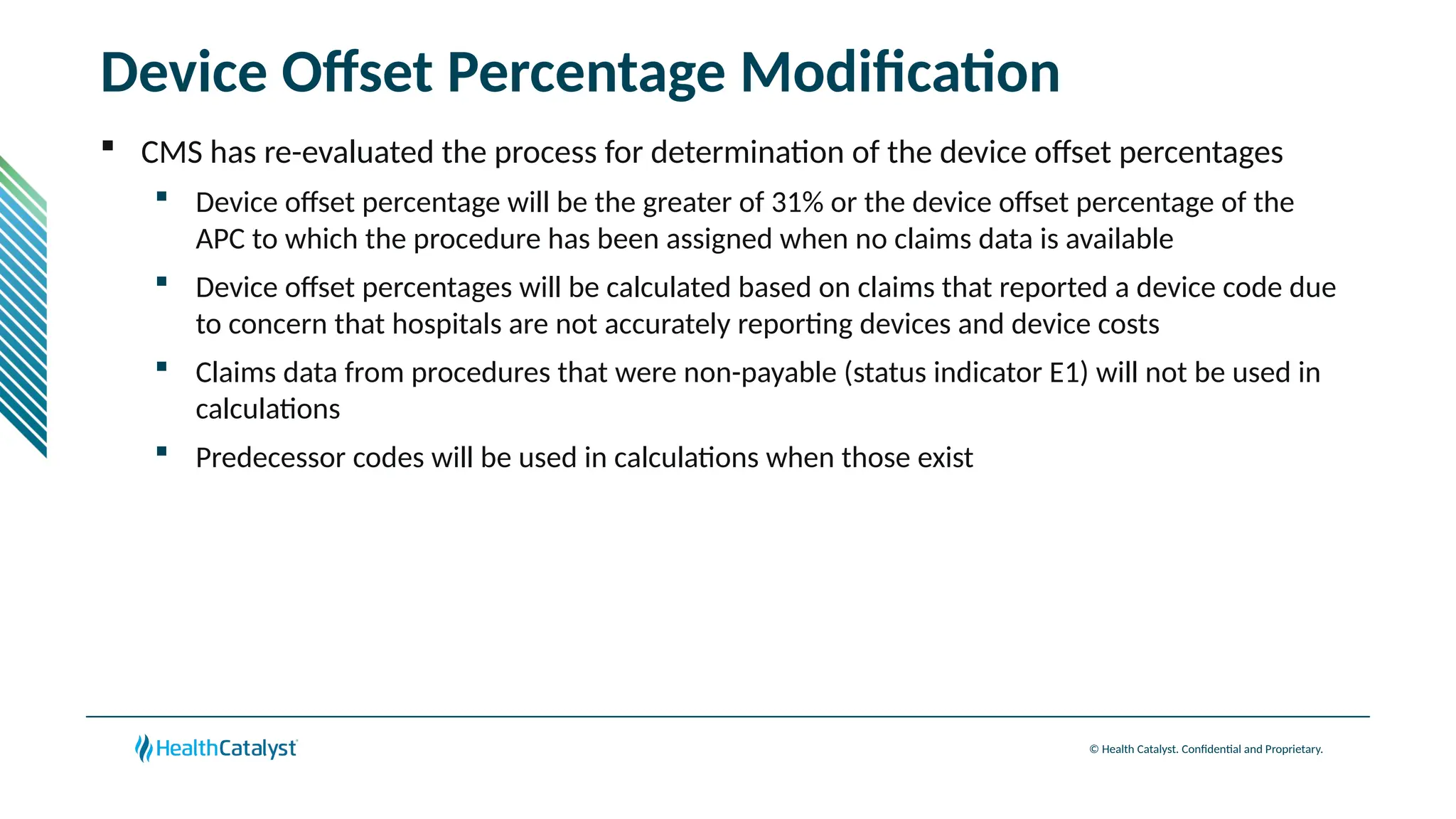
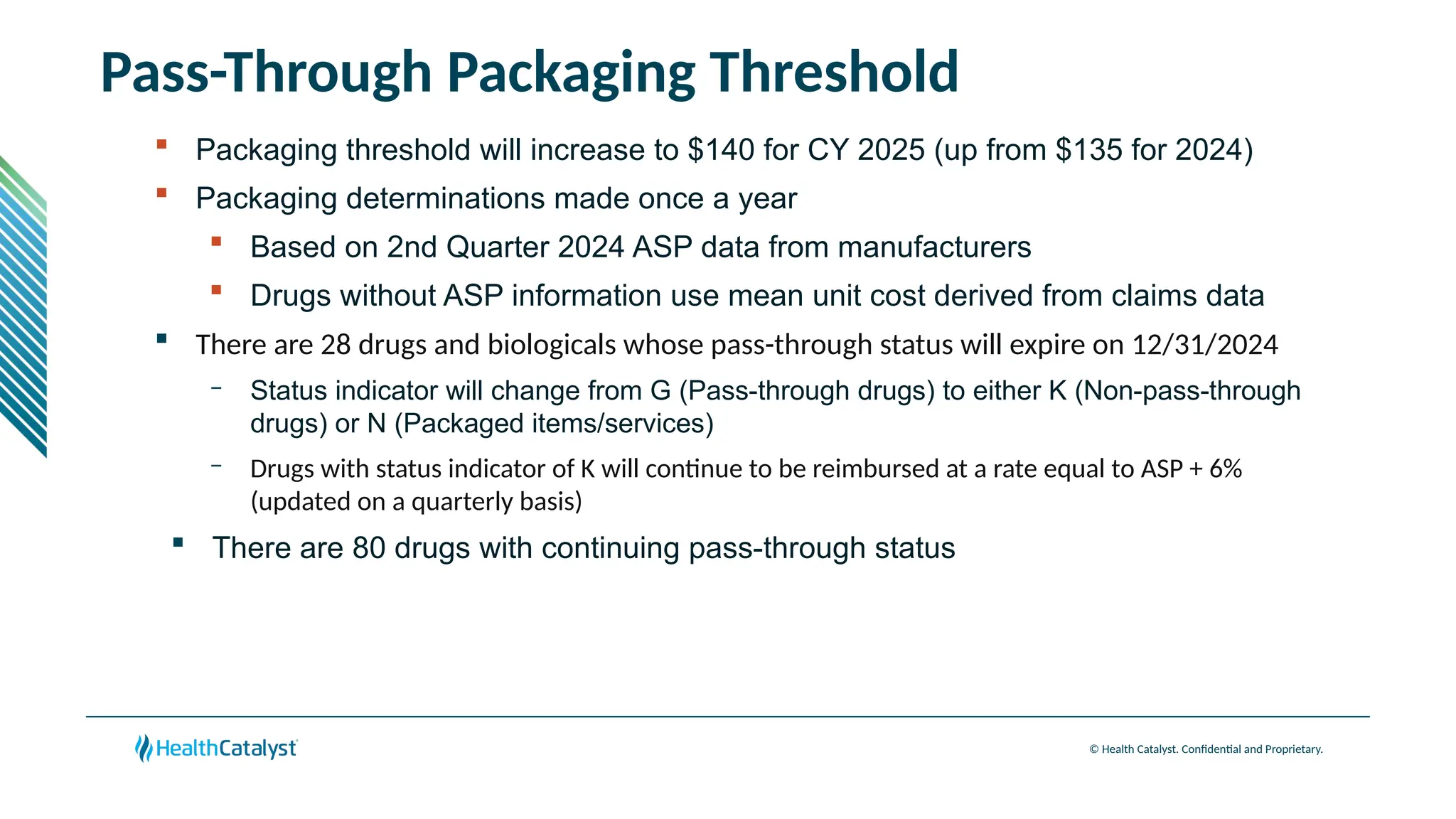
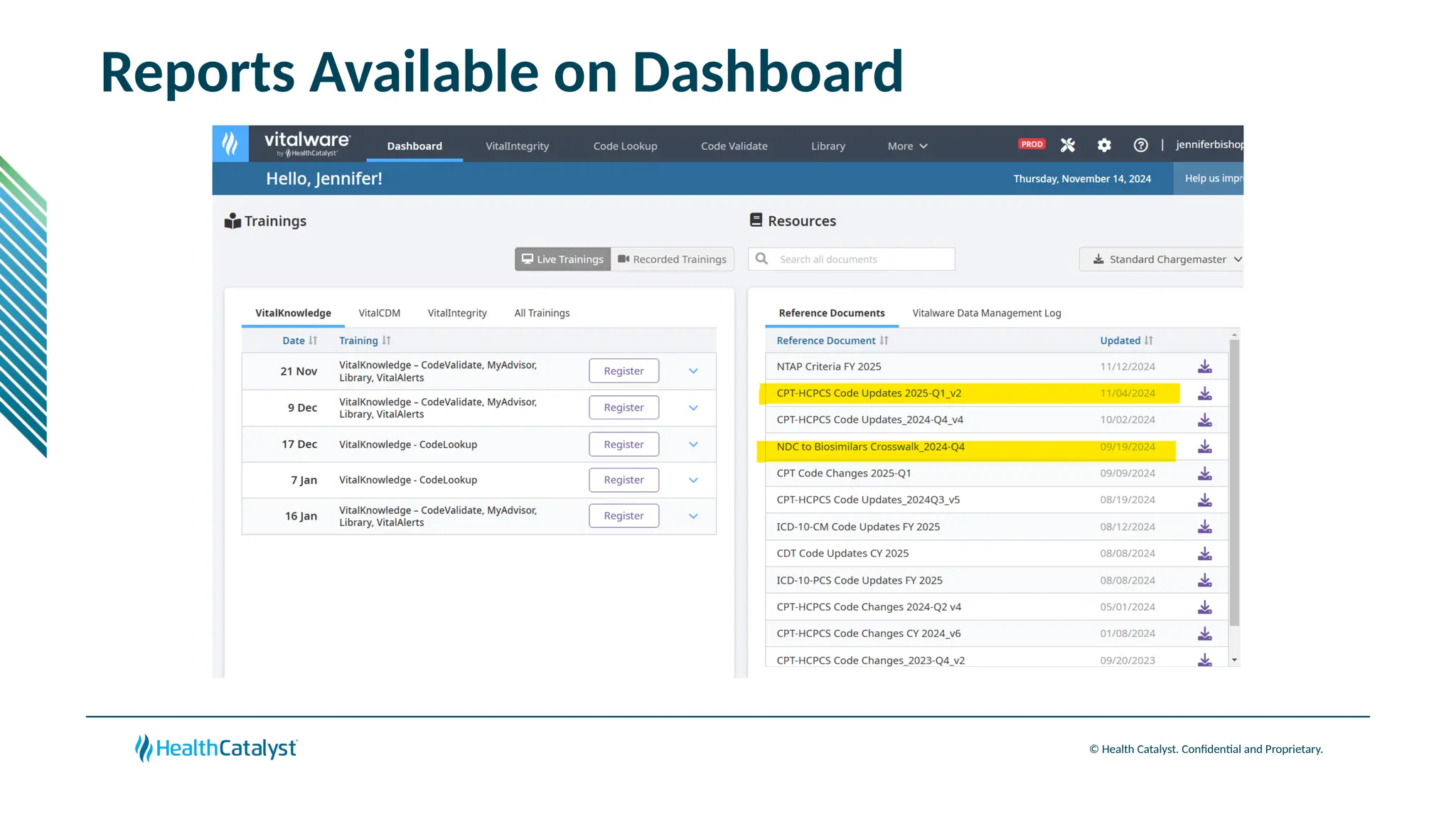
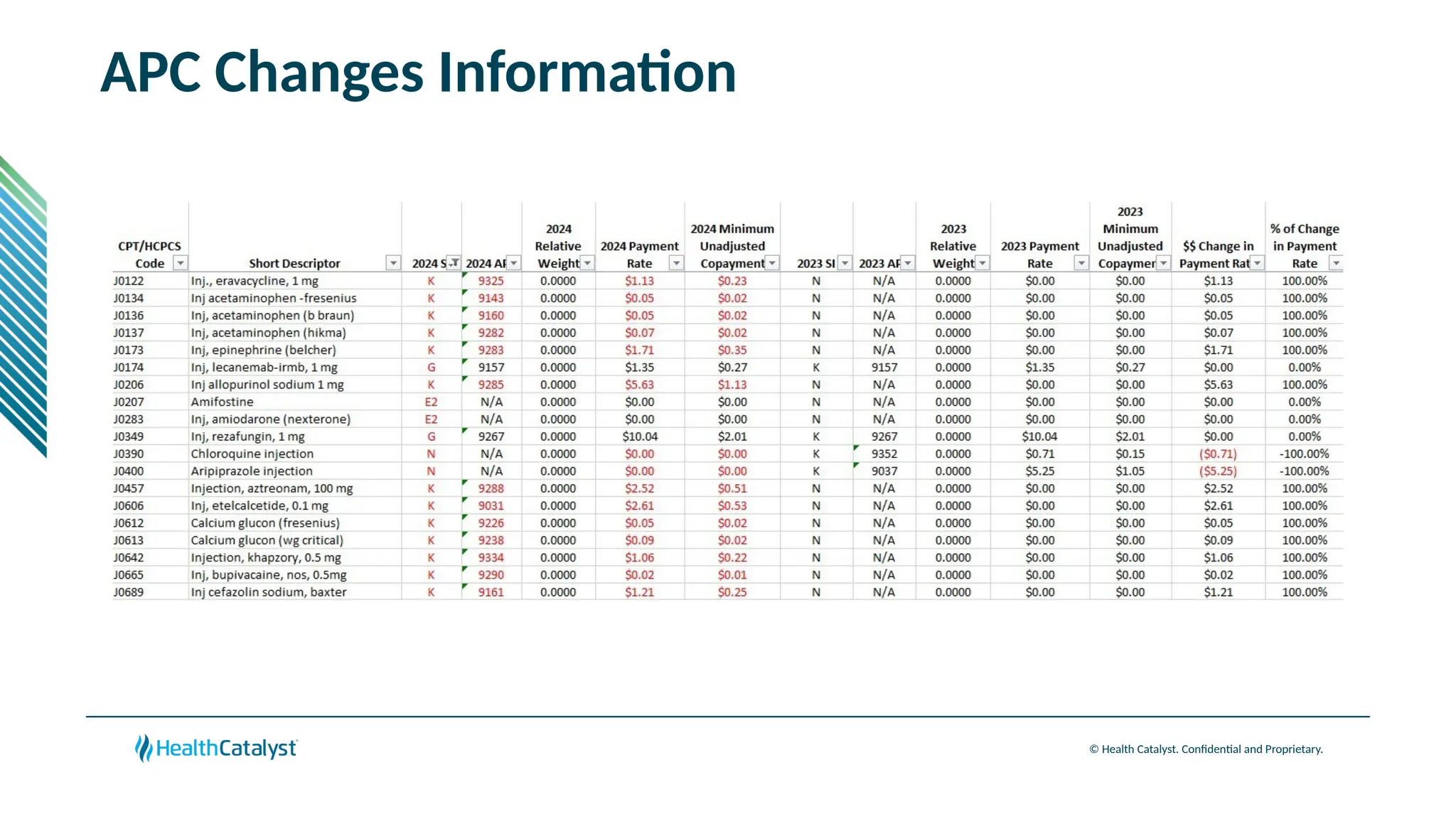
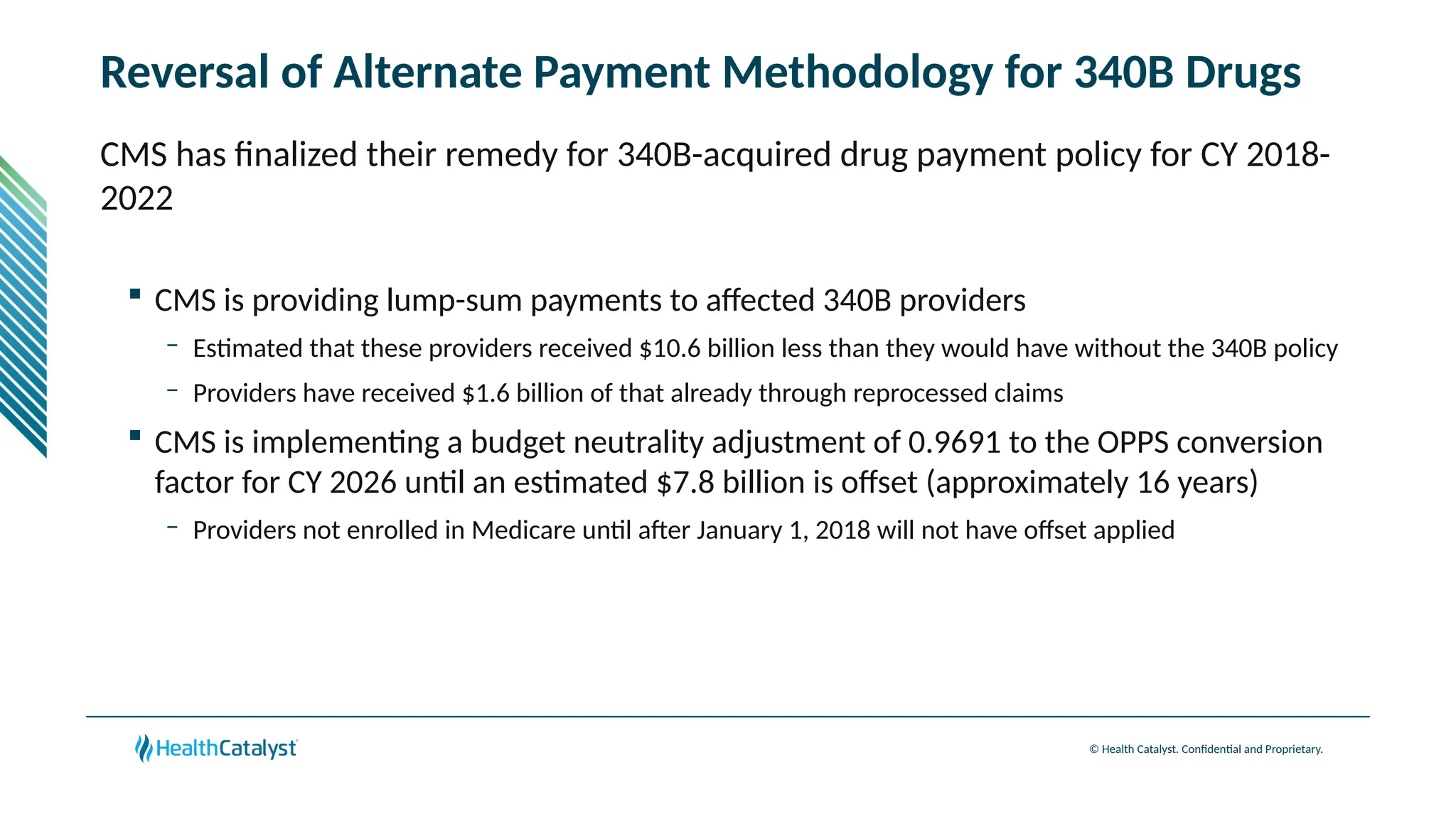
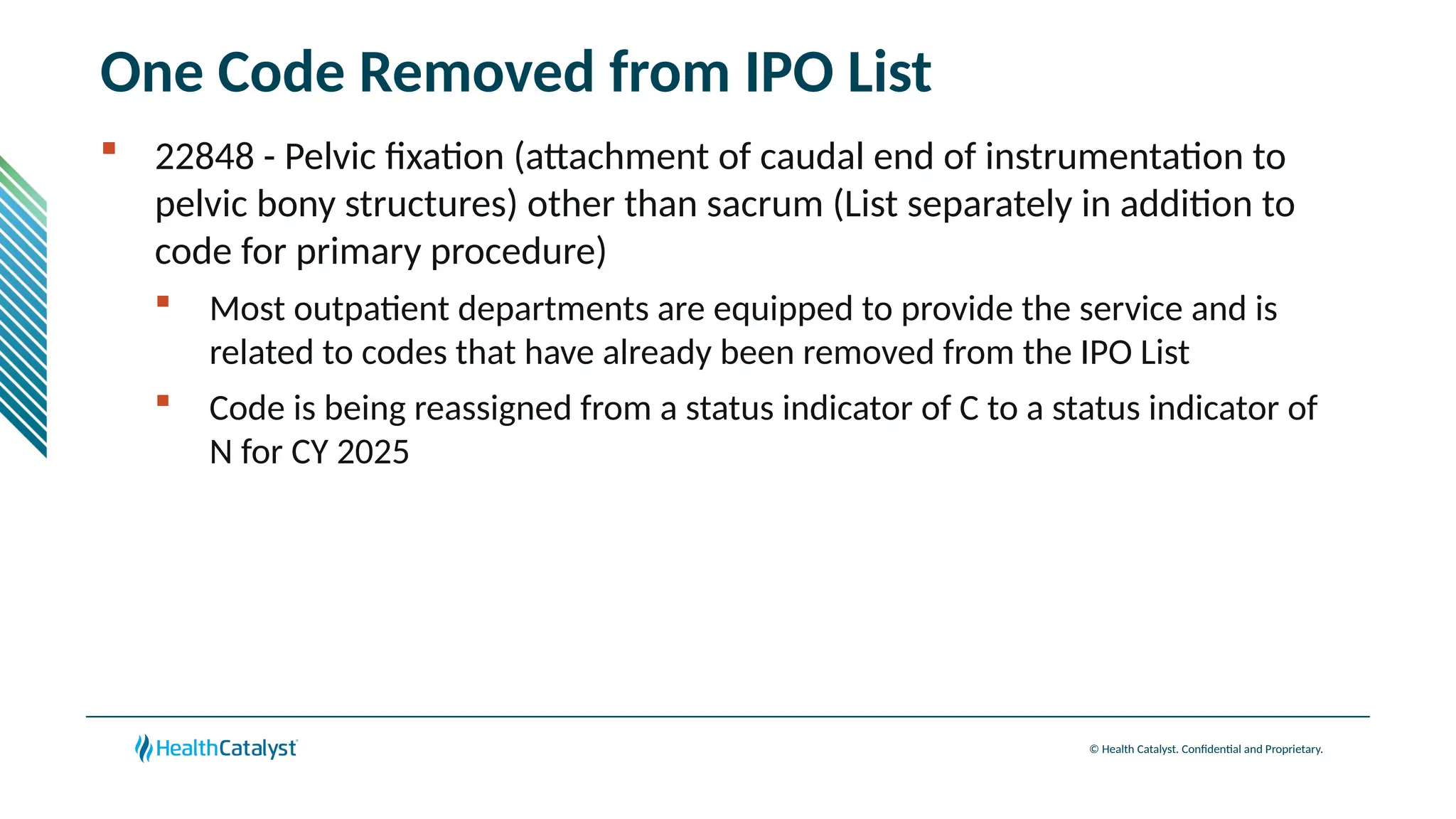
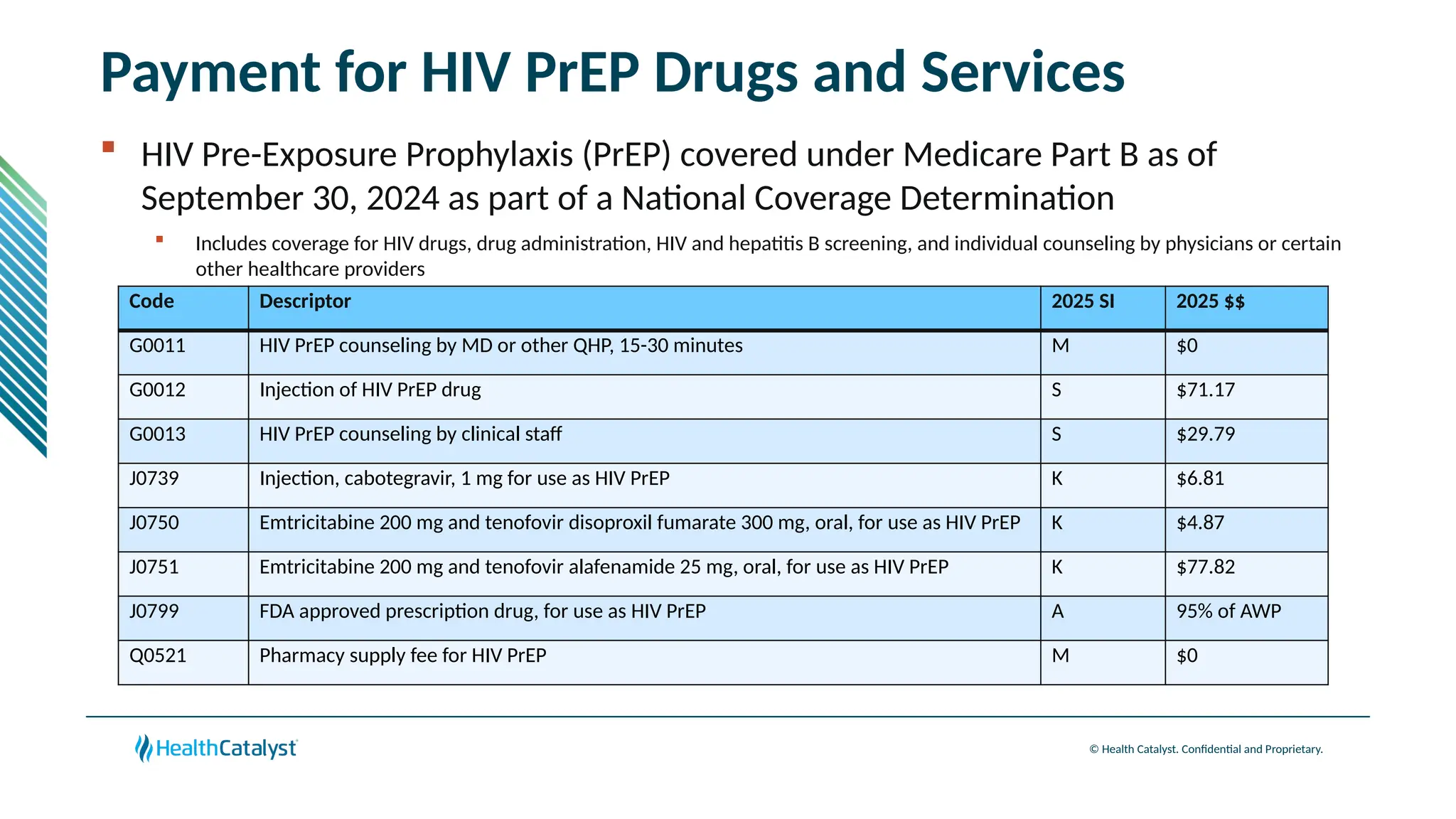
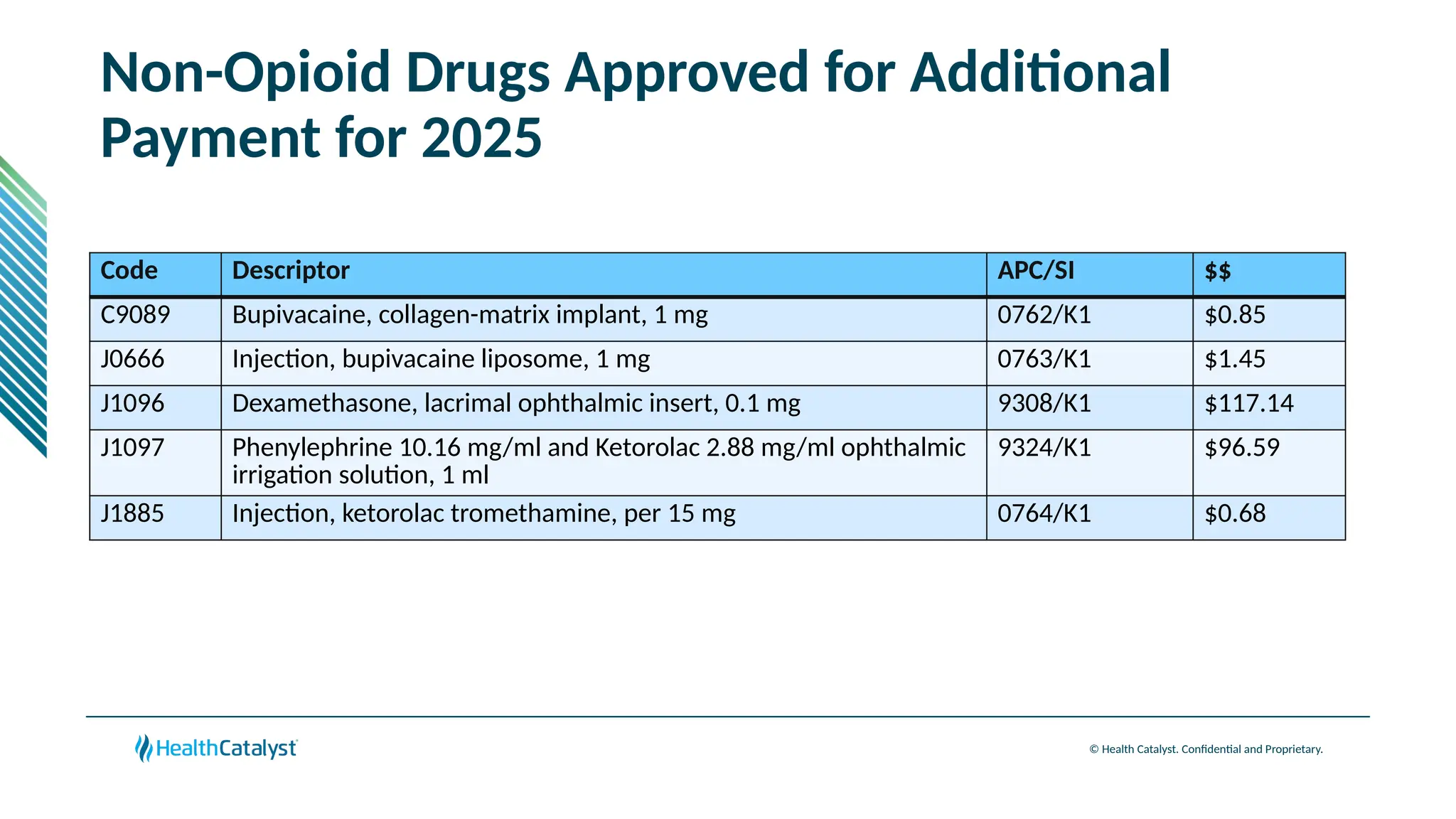
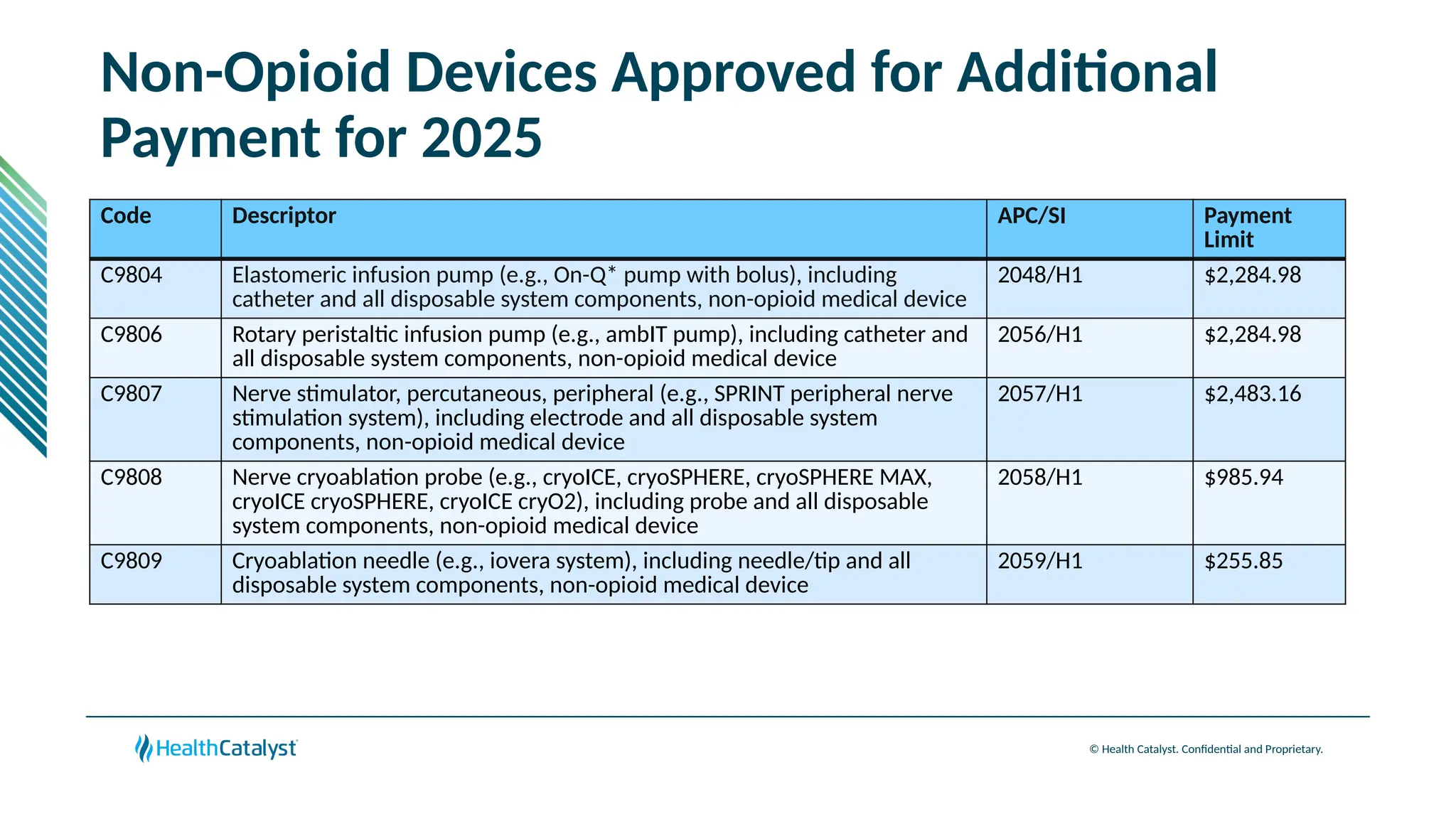
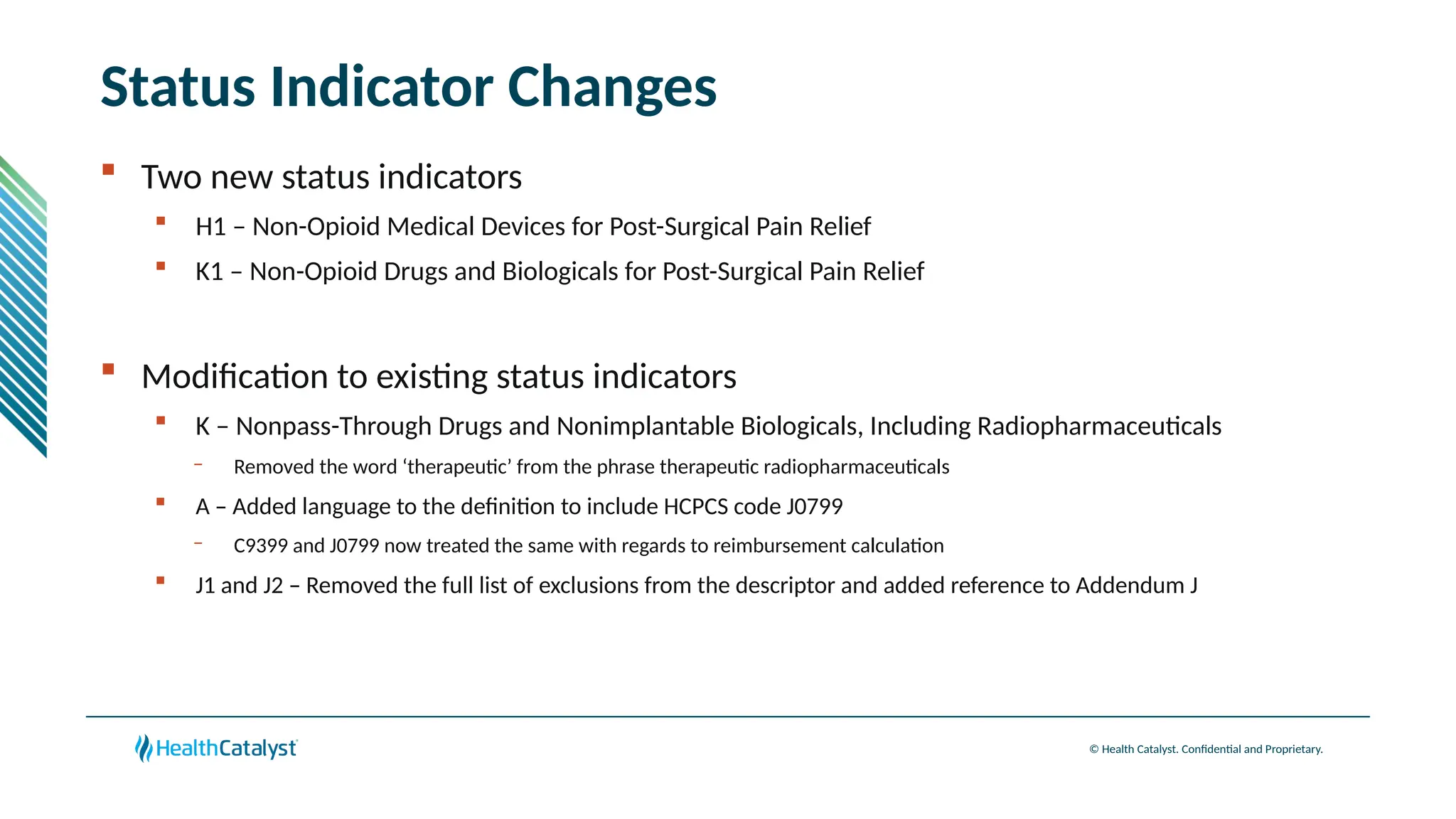
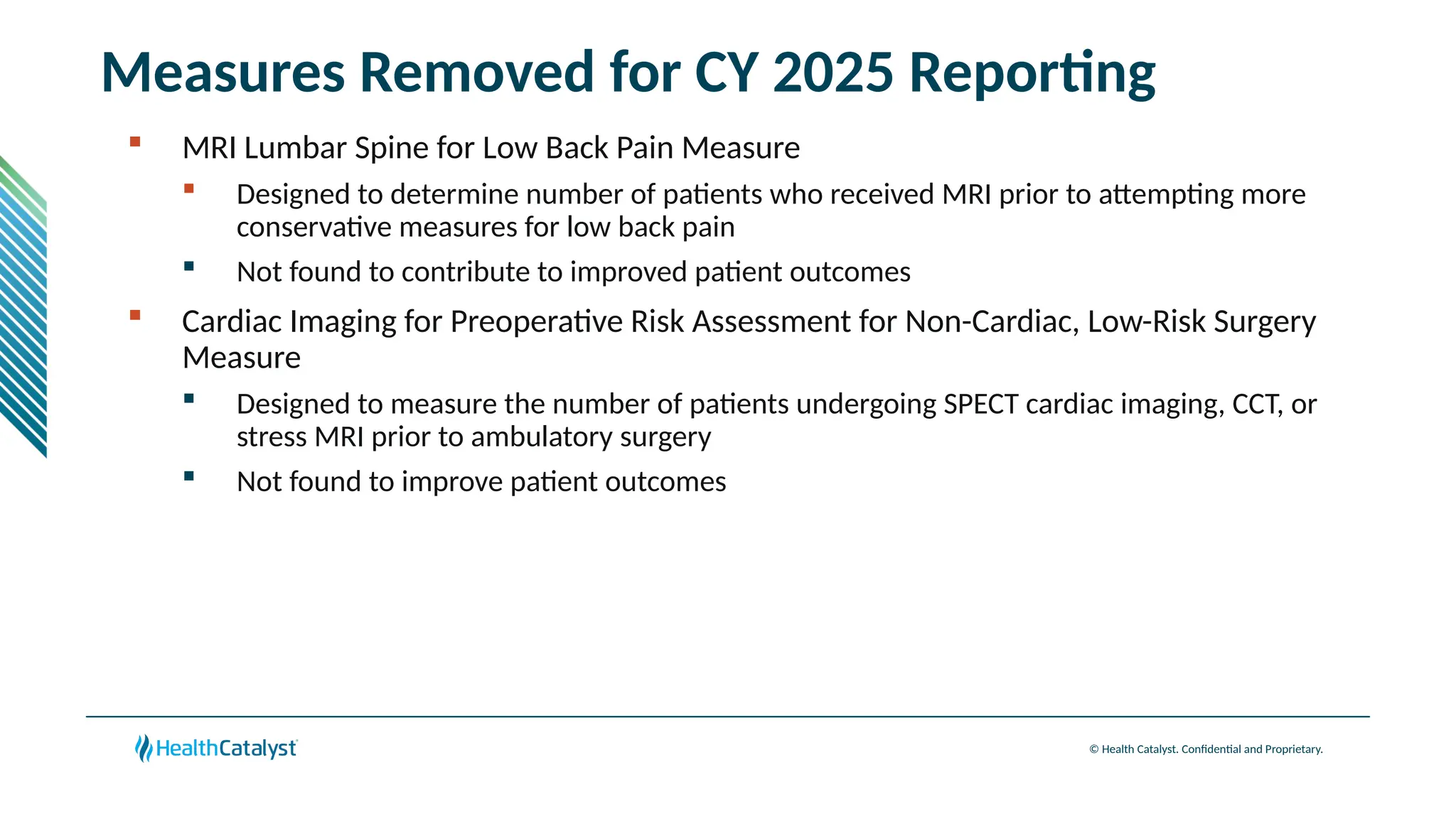
The document provides an overview of updates to the Outpatient Prospective Payment System (OPPS) for 2025, including an increase in the conversion factor to $89.169 and a predicted 3.2% rise in payments to providers. Key changes include adjustments for hospital outlier payments, new exclusions from packaging for cell and gene therapies, and several updates to specific service reimbursement rates. Additionally, new technology codes and their associated payment adjustments are detailed, illustrating how certain procedures will be billed and reimbursed under the new rules.