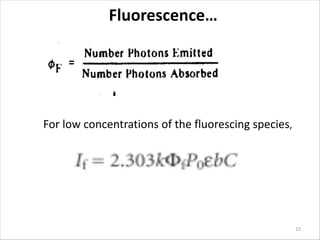
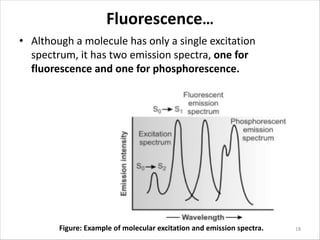
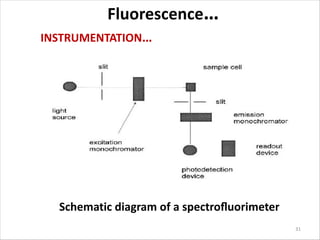
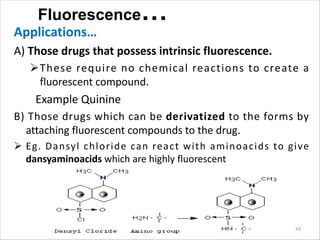
Fluorescence spectroscopy is a technique that uses fluorescence from molecules to analyze samples. Certain molecules emit light at longer wavelengths after absorbing ultraviolet or visible light (fluorescence). This technique is highly sensitive and can detect fluorescent compounds even when present at low concentrations. It has various applications like determining drugs in formulations, studying drug-protein binding, and bioanalysis. Factors like temperature, pH, concentration, and molecular structure can influence fluorescence intensity. Fluorometers contain a light source, wavelength selection devices, and photodetectors to measure fluorescence from samples.