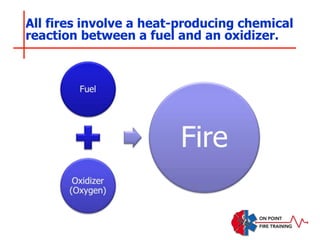
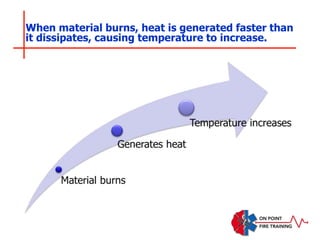
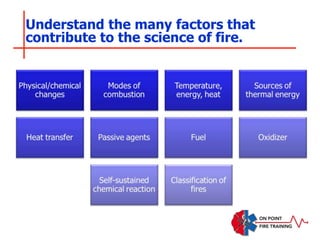
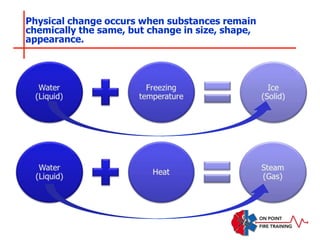
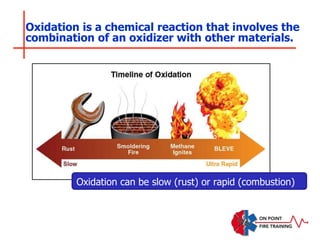
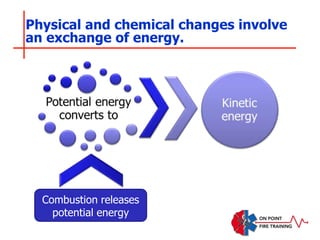
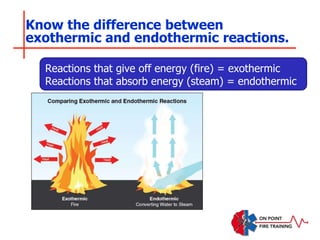
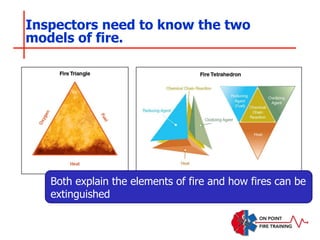
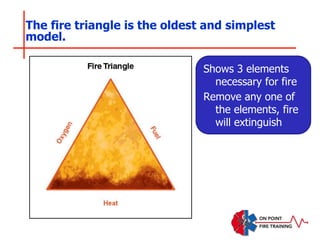
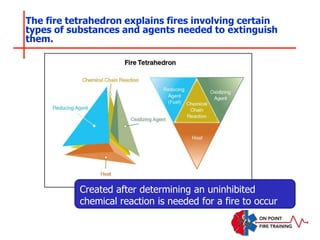
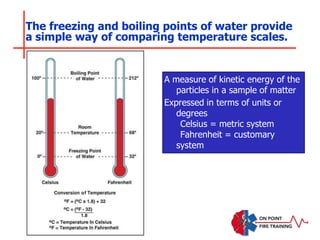
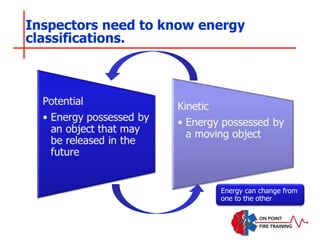
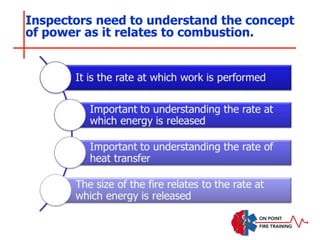
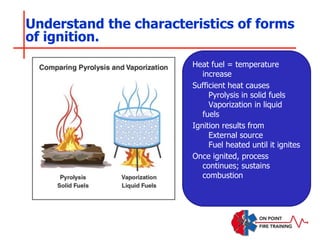
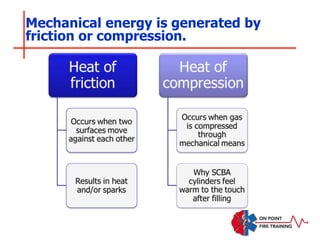
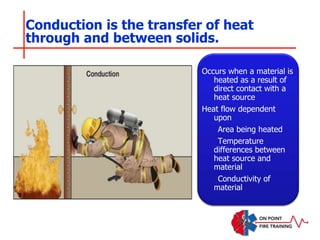
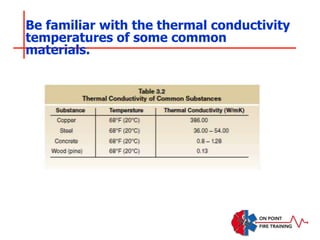
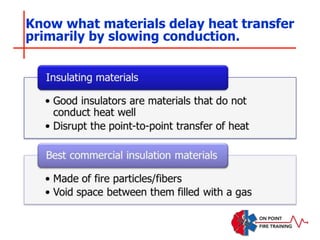
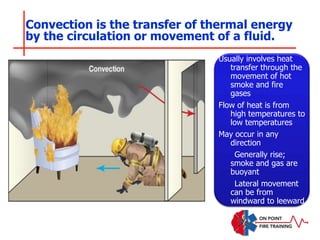
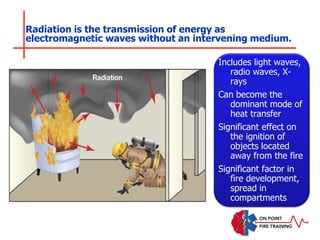
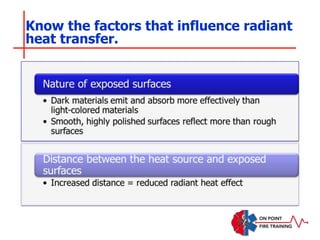
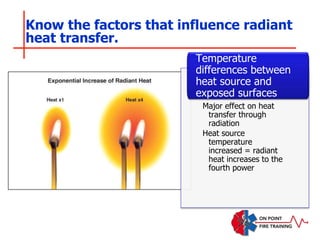
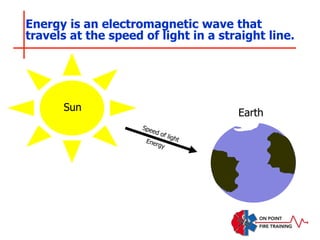
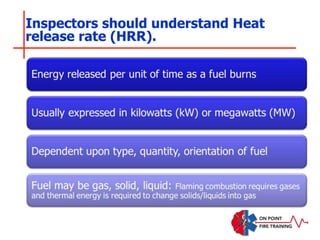
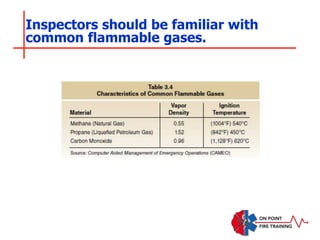
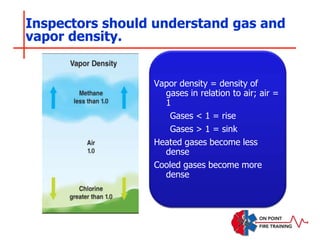
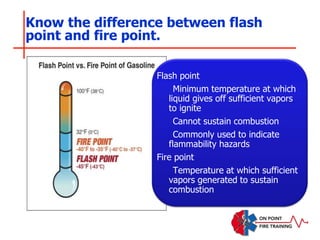
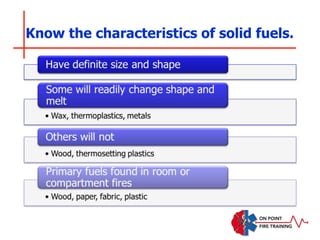
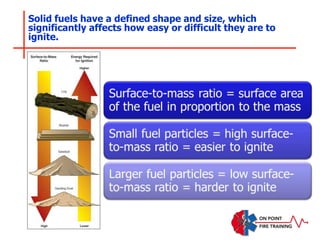
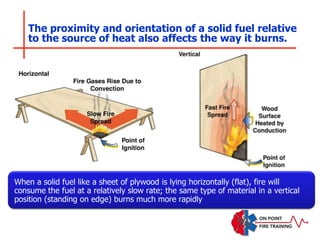
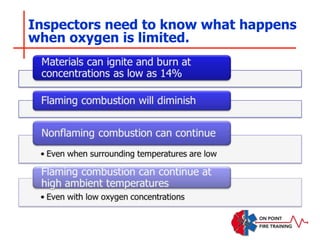
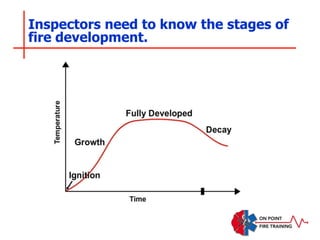
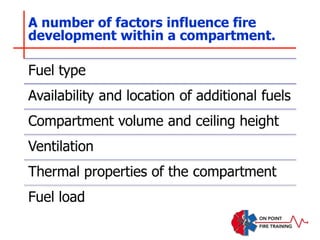
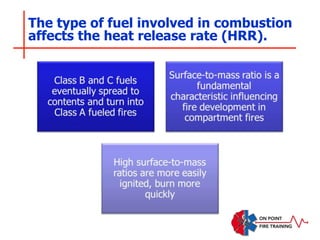
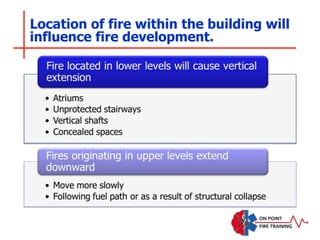
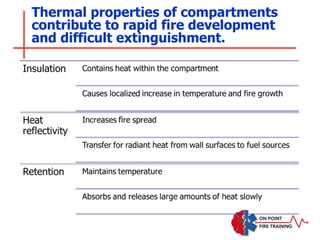
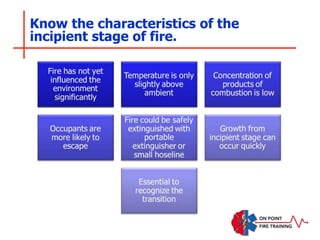
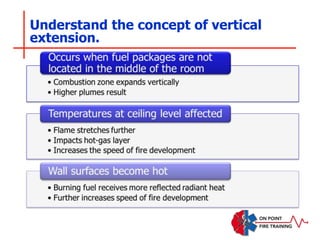
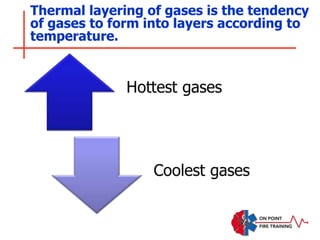
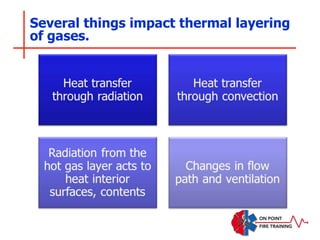
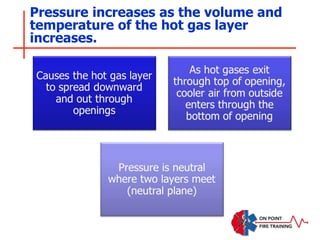
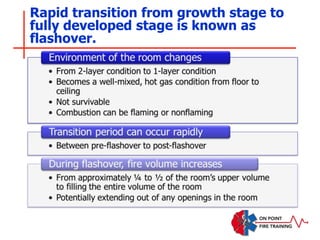
The document discusses fire behavior and development through various stages. It describes the components of fire, including the fire triangle and tetrahedron models. The stages of fire are defined as incipient (beginning), growth, fully developed, and decay. Factors that influence fire development within a compartment include available fuels, ventilation, and thermal properties. Flashover can occur when the fire rapidly transitions from growth to fully developed stage.