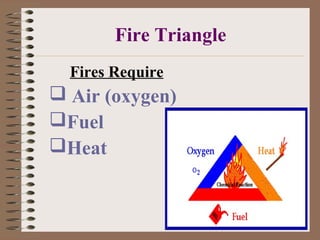
The document discusses the chemistry of fire, including the fire triangle of oxygen, fuel, and heat required to start a fire. It explains the four classes of fires based on the type of fuel (A, B, C, D) and defines key terms like pyrolysis, combustion, activation energy, and chain reaction. Fire spread occurs primarily through conduction, convection and radiation. As the fire grows, it can lead to a flashover where the flames spread rapidly to involve the entire compartment.