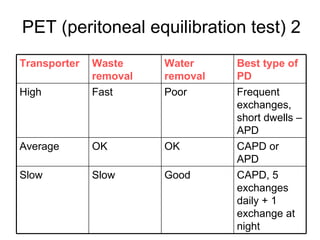
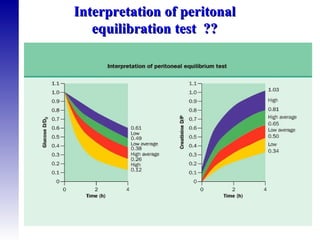
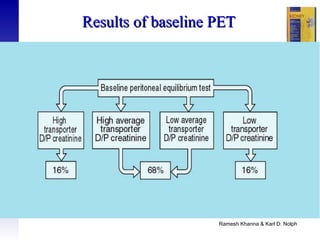
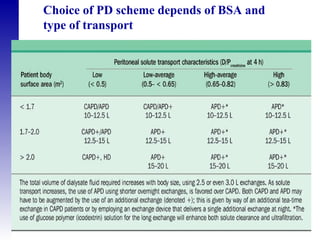
This document provides an overview of peritoneal dialysis, including:
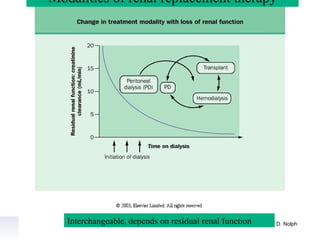
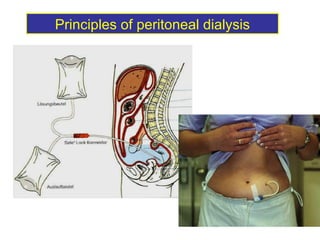
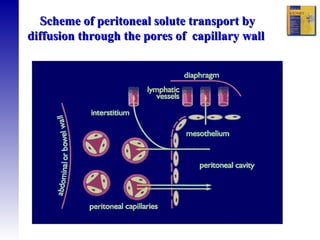
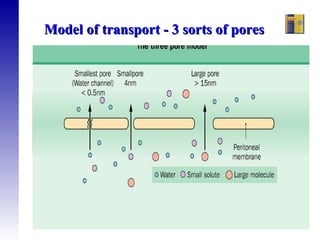
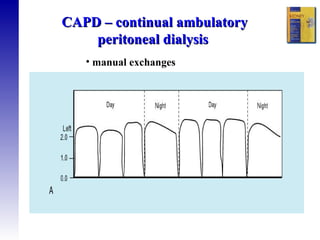
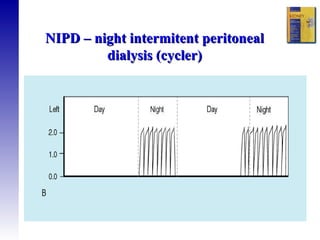
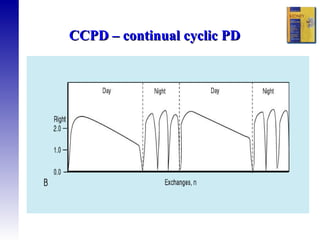
1. The principles of peritoneal dialysis, which uses the peritoneum as a semipermeable membrane for diffusion and convection of fluids and solutes.
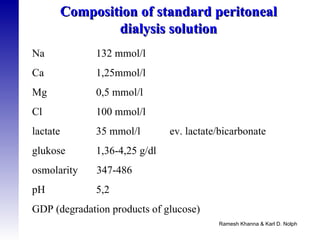
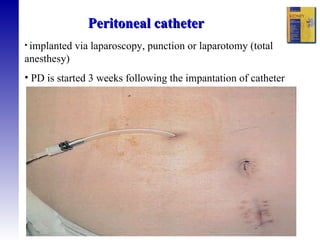
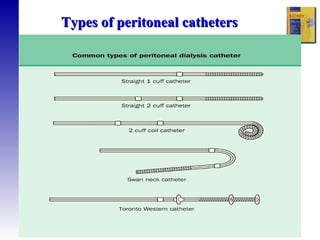
2. The types of peritoneal dialysis solutions and catheters used, as well as factors that influence ultrafiltration.
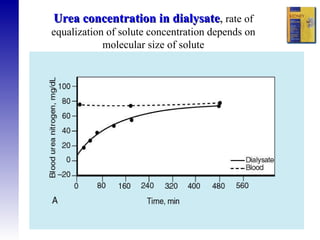
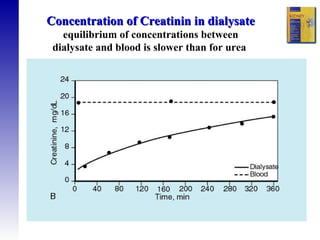
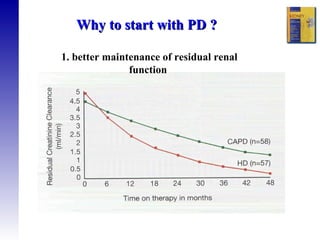
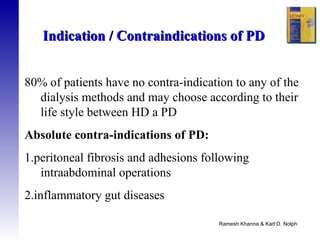
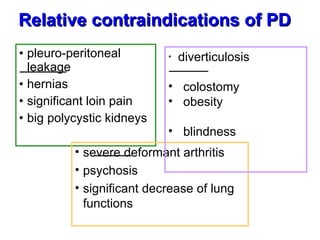
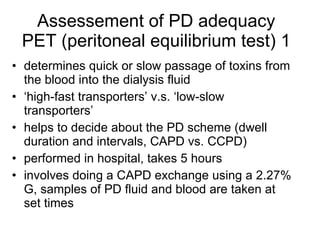
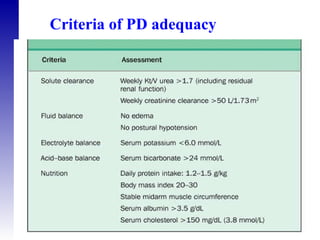
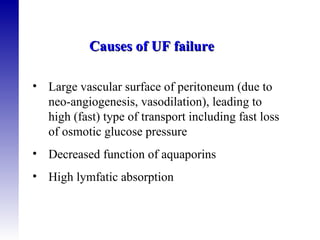
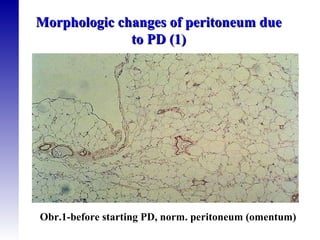
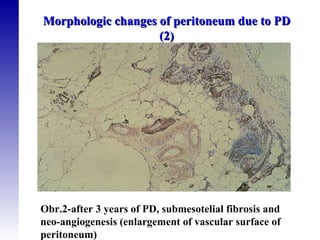
3. Methods for assessing peritoneal function and dialysis adequacy, along with the complications that can arise with long-term peritoneal dialysis.