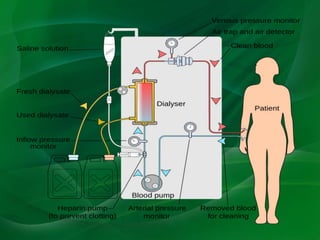
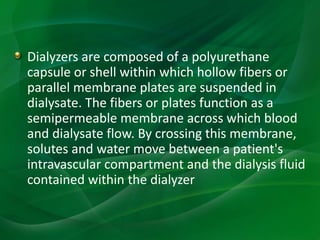
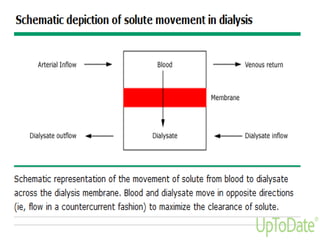
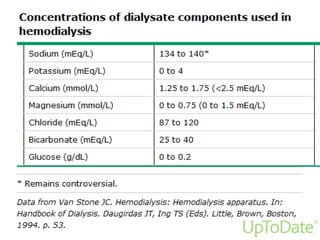
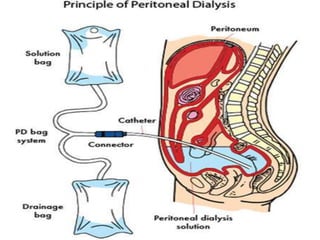
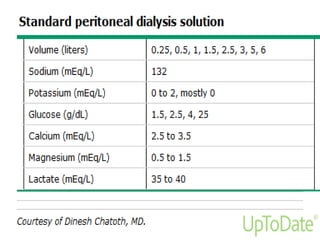
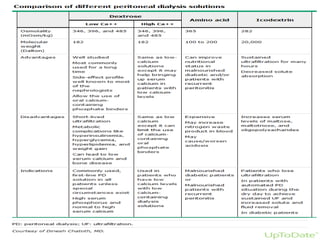
This document provides information on hemodialysis, peritoneal dialysis, and kidney transplantation for treatment of end-stage renal disease. It discusses how hemodialysis uses an external device to filter waste from the blood and how peritoneal dialysis uses the peritoneal membrane for filtration. Complications of each method like peritonitis and access issues are also outlined. The document concludes with a brief discussion of kidney transplantation eligibility criteria and processes.