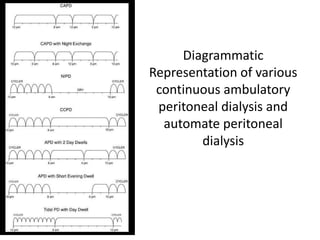
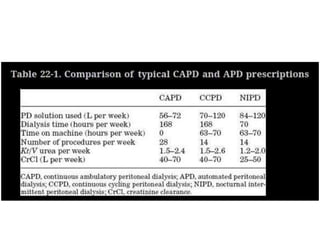
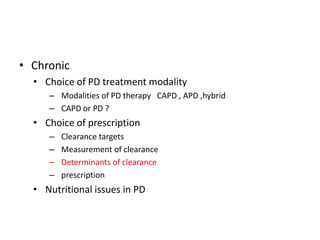
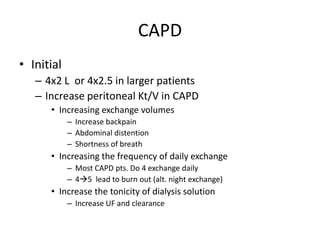
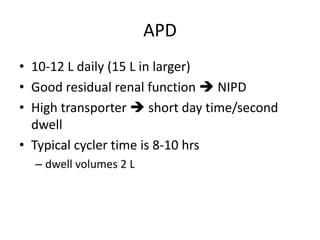
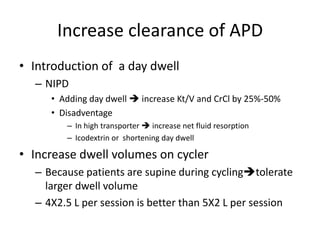
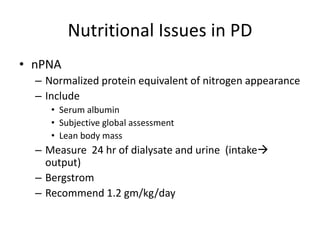
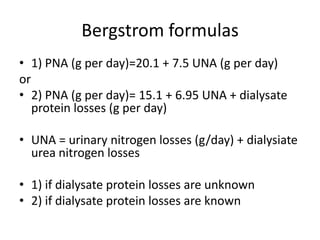
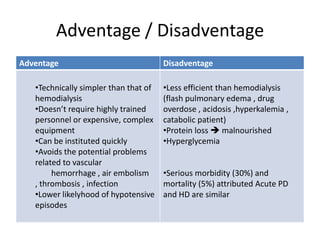
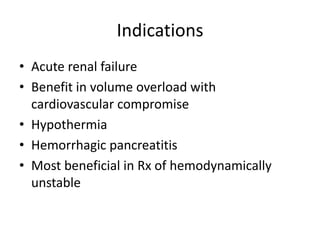
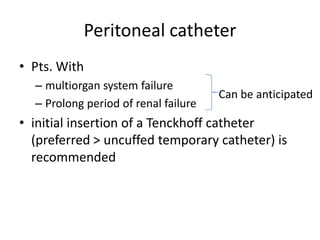
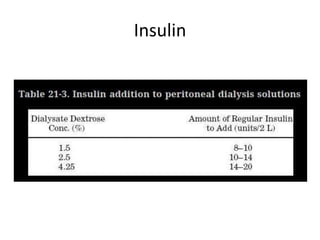
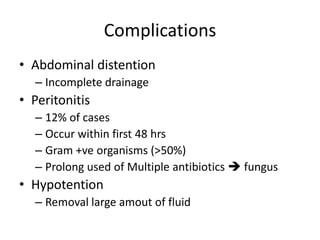
This document discusses prescribing acute and chronic peritoneal dialysis. For acute PD, it recommends using a Tenckhoff catheter and automated cyclers. Exchanges should be hourly with 2L volumes. Clearance is monitored using BUN levels and D:P ratios. Complications include abdominal distention and peritonitis. For chronic PD, clearance targets are a Kt/V of 1.7 per week. Prescriptions are based on residual renal function, transporter status, and body size. CAPD and APD are both options depending on lifestyle. Clearance can be increased by optimizing exchange volumes, frequency, and solution tonicity.











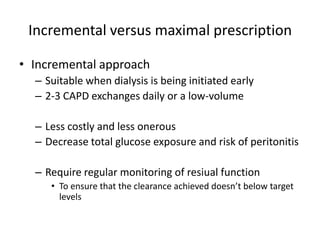
![Exchange time
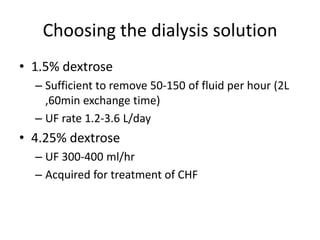
• Dwell period
• Standard dwell period
– Usual dwell time is 30 min
– 2L per exchage 48 L per day
– [Urea] in drained dialysate will be 50-60% of plasma
• More stable patients
– If Not extremely hypercatabolic state
• longer dwell time 1.5-5 hrs
– At 5 hrs [UREA] dialysate = [UREA]plasma](https://image.slidesharecdn.com/apd-121019111647-phpapp02/85/PD-prescription-19-320.jpg)



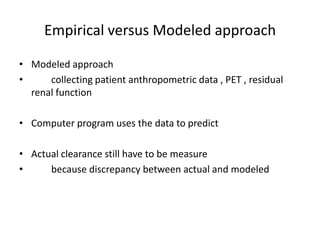
![Monitor Clearance
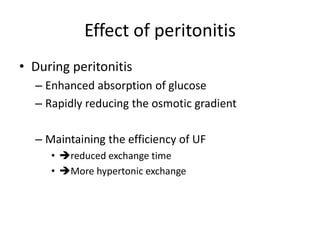
• In general
– BUN should maintain below 80 mg/dl
– D:P ratio for urea
• [BUN]dialysate : [BUN]plasma ratio
• Multiplied by total daily dialysate volume urea daily
clearance
• Should be at least 10 ml/min
• 20-30 ml/min in hypercatabolic patient](https://image.slidesharecdn.com/apd-121019111647-phpapp02/85/PD-prescription-28-320.jpg)

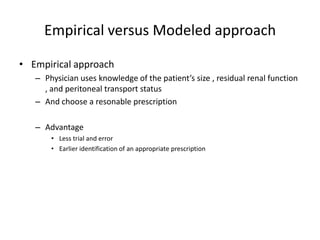
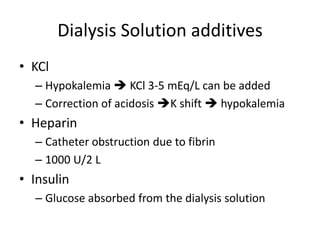
![Complications
• Hyperglycemia
– IP insulin
• Hypernatremia
– UF generated in PD [Na] 70 mEq/L
– Increased loss of water
• Hypoalbuminemia
– Protein loss 10-20 gm /day
– Early oral or parenteral hyperalimentation should
be instituted](https://image.slidesharecdn.com/apd-121019111647-phpapp02/85/PD-prescription-31-320.jpg)