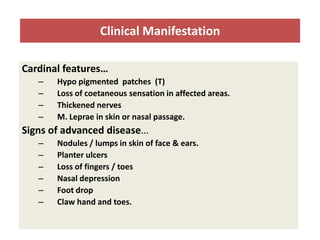
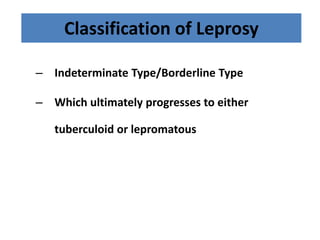
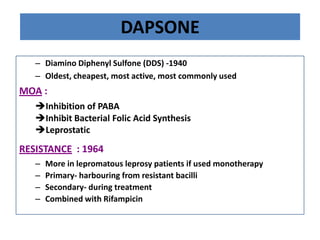
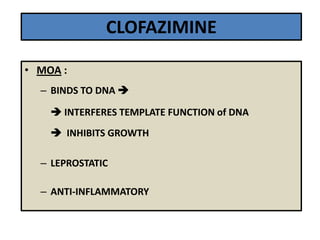
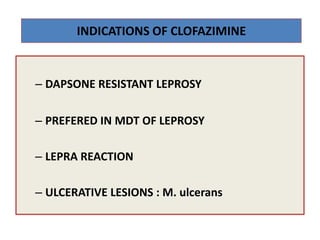
This document discusses leprosy (Hansen's disease), including its epidemiology, clinical manifestations, bacteriological classification, and chemotherapy. It is caused by Mycobacterium leprae. The main drugs used to treat leprosy are dapsone, clofazimine, rifampin, ofloxacin, clarithromycin, and minocycline. The World Health Organization recommends either a 6-month multi-drug regimen for paucibacillary leprosy or a 2-year multi-drug regimen for multibacillary leprosy. The document reviews the mechanisms of action, pharmacokinetics, indications, and adverse effects of the main antileprotic drugs.