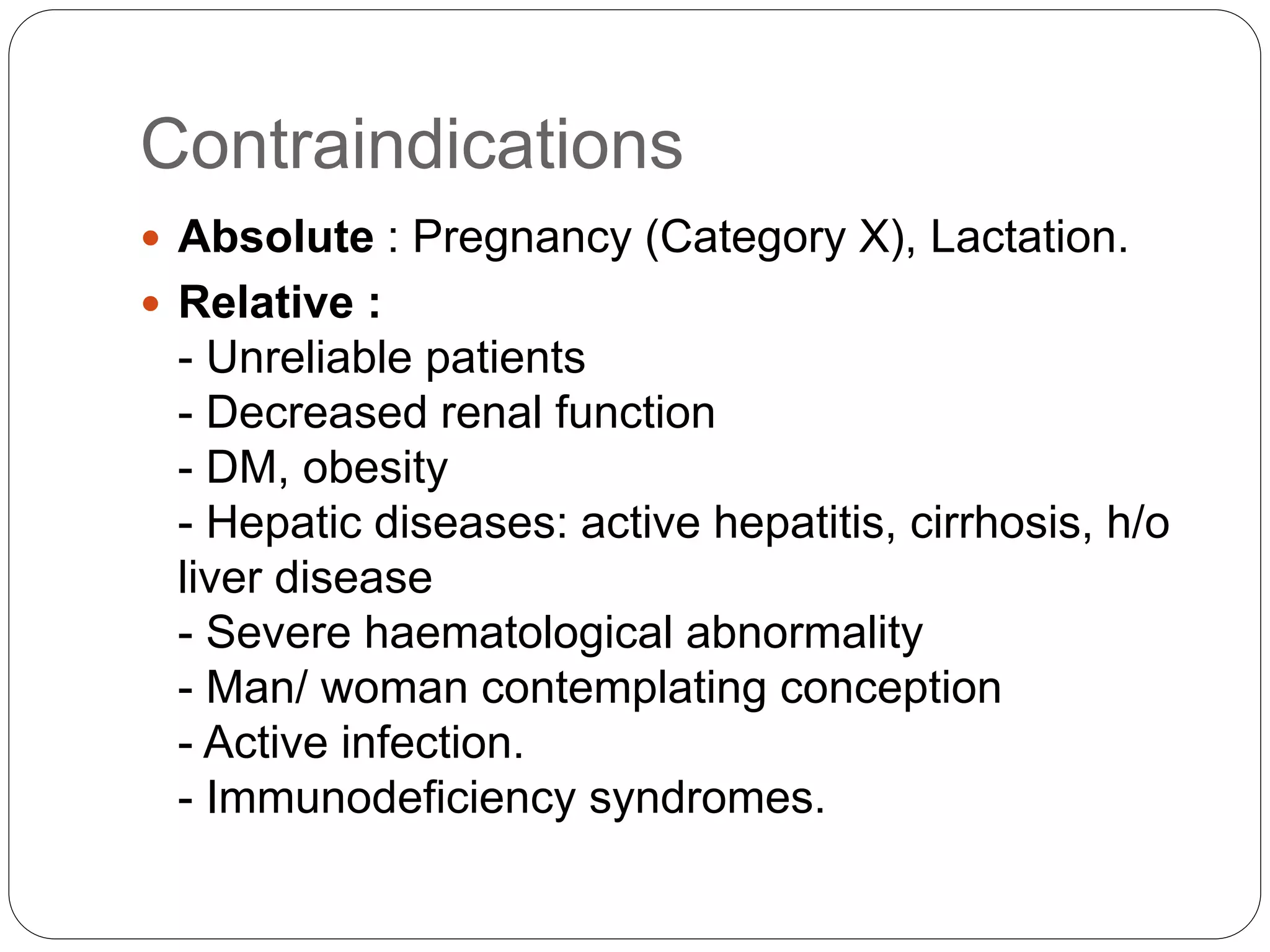
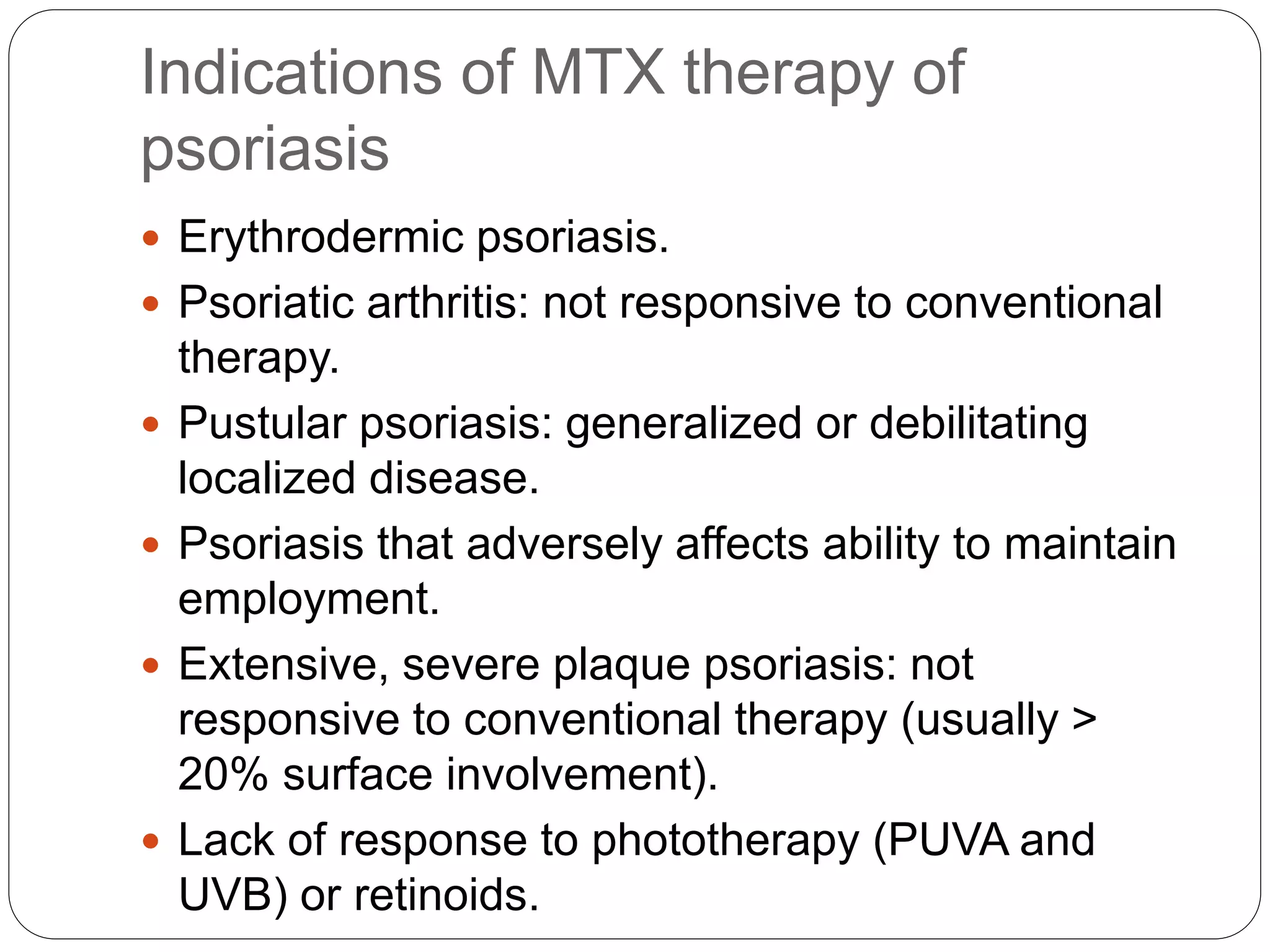
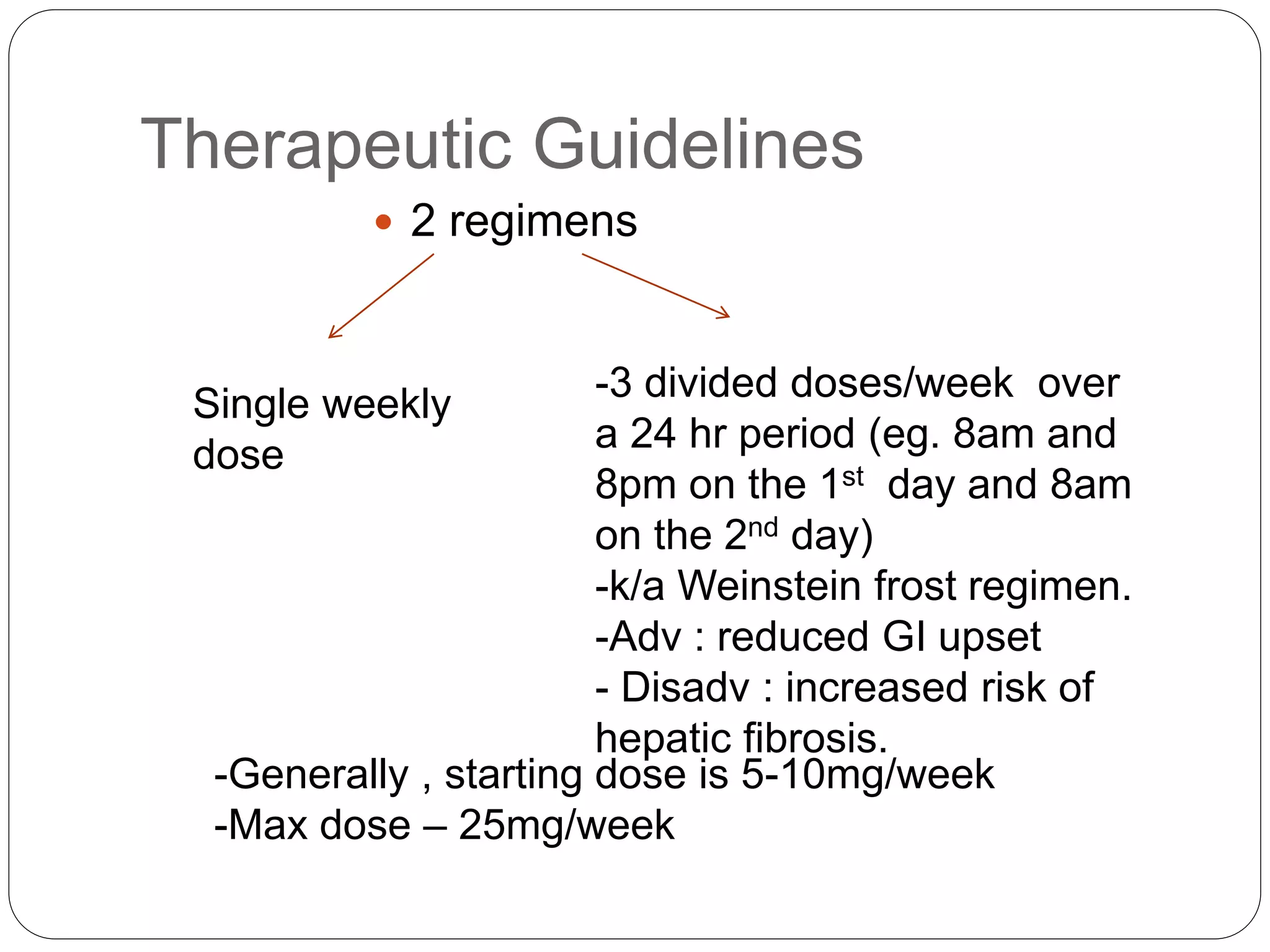
Methotrexate is a folic acid analogue that inhibits dihydrofolate reductase and is used to treat various inflammatory and proliferative skin conditions. It can be administered orally, intravenously, intramuscularly or subcutaneously. It is well distributed throughout the body except in the brain. Around 50% is bound to plasma proteins and it has a terminal half-life of 10-27 hours. It works by inhibiting DNA synthesis and blocking T cell migration. Common side effects include gastrointestinal upset and hepatotoxicity. It is contraindicated in pregnancy due to risk of teratogenicity. Monitoring for toxicity and supplementing with folic acid can help reduce adverse effects.