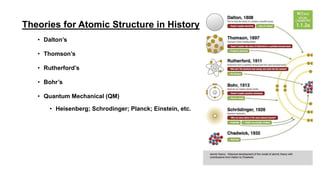
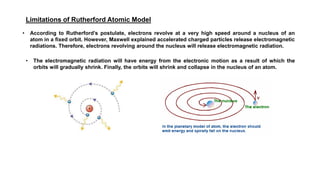
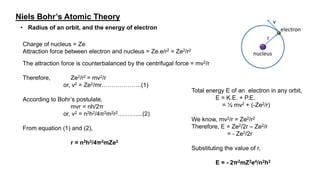
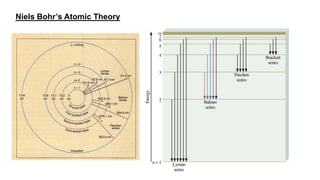
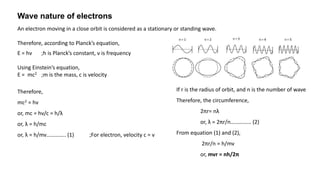
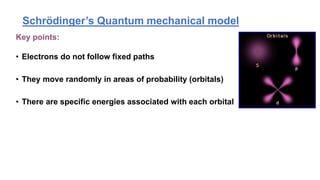
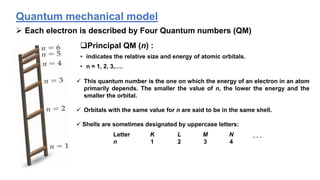
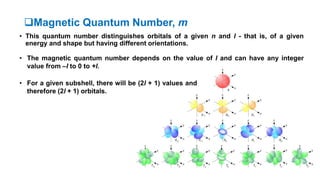
The document discusses the history of atomic structure theories including Dalton's, Thomson's, Rutherford's, Bohr's, and the quantum mechanical model. Dalton proposed that atoms are indivisible and compounds form by combination of atoms. Rutherford's gold foil experiment showed that the positive charge and mass of an atom are concentrated in a small nucleus. Bohr incorporated Planck's quantum theory, proposing electrons orbit in discrete energy levels. Later, the quantum mechanical model treated electrons as waves based on de Broglie's idea and Schrodinger's wave equation.










![Exceptions to the Aufbau Rule
Cr [Ar] 3 d44s2 Cu [Ar] 3 d94s2
However, the actual electron configuration, determined
experimentally, are:
Cr [Ar] 3d54s1 Cu [Ar] 3d104s1](https://image.slidesharecdn.com/atomicstructures-dr-220907051840-db663976/85/Atomic-Structures-Dr-Mahbub-pptx-29-320.jpg)