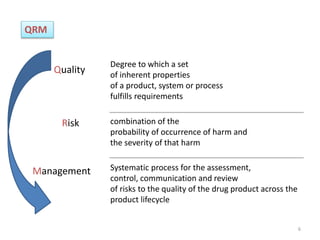
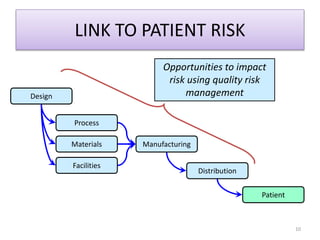
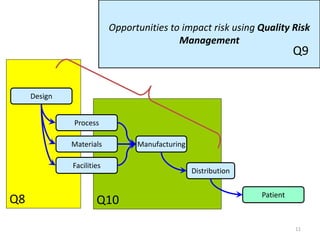
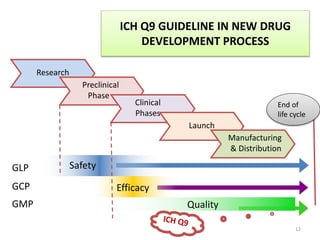
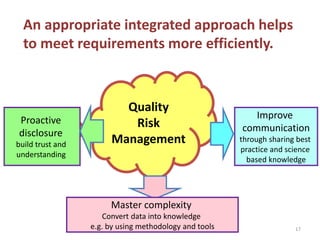
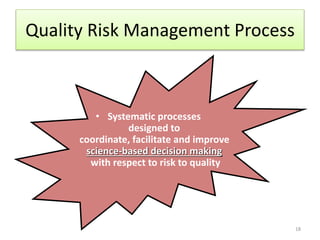
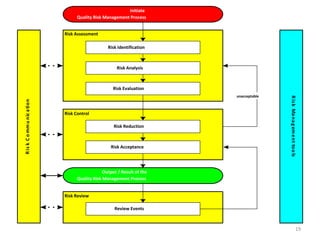
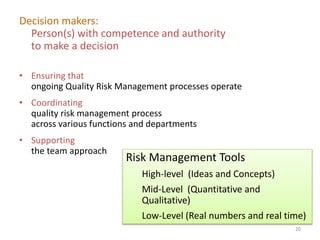
This document summarizes a presentation on ICH Guideline Q9 on quality risk management. The objectives are to understand the concept of quality risk management, risk, and ICH Q9's role in new drug development. It discusses quality risk management as a systematic process to assess, control, communicate and review risks to drug quality across the product lifecycle. ICH Q9 provides principles and tools for quality risk management that can be applied at various stages including development, manufacturing, and distribution. It emphasizes linking quality risk management activities to protecting patient safety.