Embed presentation
Downloaded 21 times







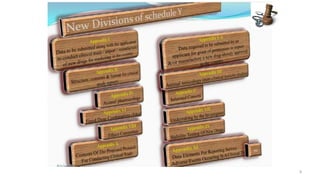
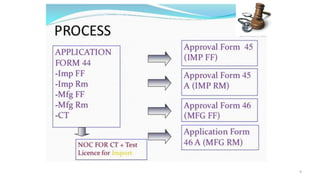



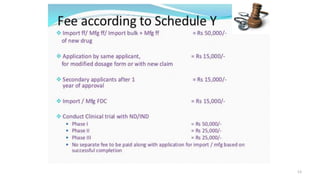











The document outlines a clinical trial presentation that aims to review and study clinical trials. The objectives are to understand the concept of clinical trials, important considerations for clinical trials in India, and the appendices of Schedule Y. Schedule Y is the main guideline for permission to import, manufacture, or conduct clinical trials of new drugs in India. It is divided into sections on important considerations for clinical trials and appendices.























