Embed presentation
Downloaded 33 times







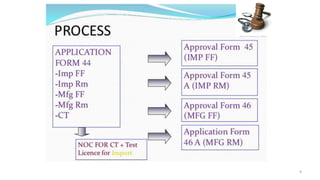



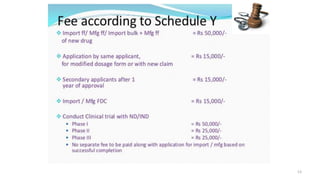











Ms. Sonali Diwate presented on reviewing and studying Schedule Y, which outlines the requirements and guidelines for permission to import, manufacture, or conduct clinical trials of new drugs in India. The objectives were to understand the concept of clinical trials, important considerations for clinical trials, and the appendices of Schedule Y. Schedule Y is divided into two main parts - important considerations for clinical trials in India, and the appendices, which provide guidelines for conducting clinical trials in compliance with regulatory standards.























