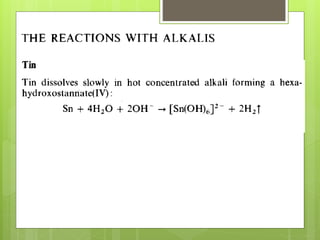
The document discusses the Group IV elements carbon, silicon, germanium, tin, and lead. Group IV elements have a full s orbital and two electrons in the p orbital. The elements range from the nonmetal carbon to the weakly metallic lead. Carbon and silicon form acidic oxides, while tin and lead form amphoteric oxides. Carbon occurs naturally as diamond and graphite. Silicon is abundant as silica but not as the free element. Germanium is a rare element found in coal products. Tin is obtained commercially from its oxide cassiterite. Lead is extracted from its sulfide ore by roasting and heating with limestone and coal in a blast furnace.