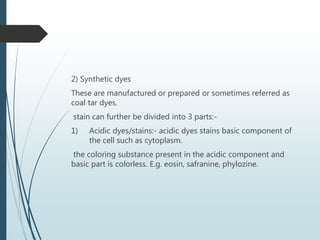
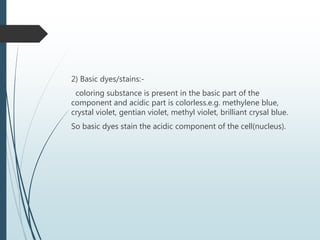
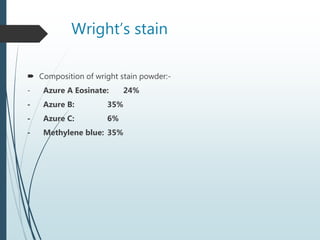
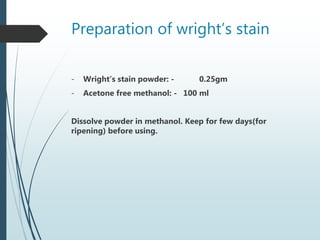
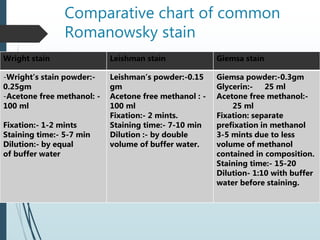
This document discusses various dyes and stains used in biological studies. It begins by distinguishing between dyes, which are manufactured substances, and stains, which are dye solutions. Dyes can be natural, extracted from sources like lichens, or synthetic and manufactured. Common stains discussed include Wright's stain, Leishman's stain, Giemsa stain, and Romanowsky stains. The document provides details on the composition and preparation of these stains as well as their staining properties and applications in studying blood smears, bone marrow, and malaria parasites.