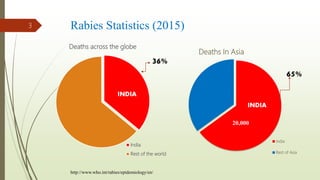
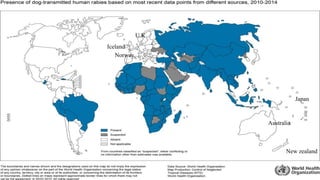
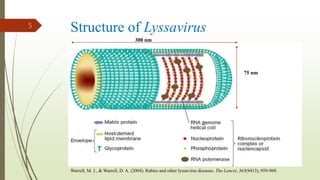
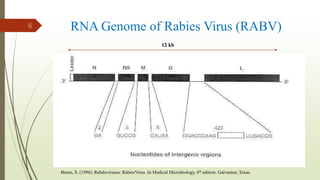
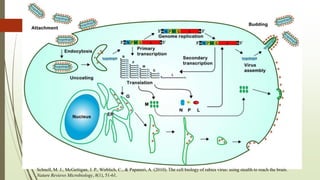
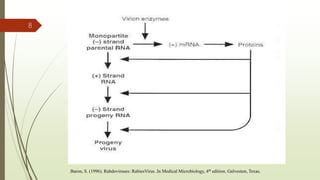
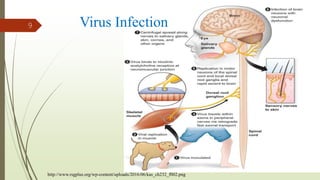
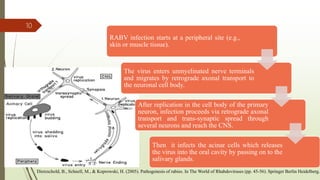
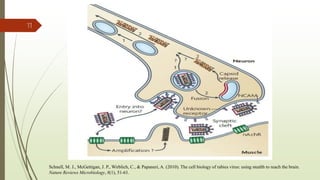
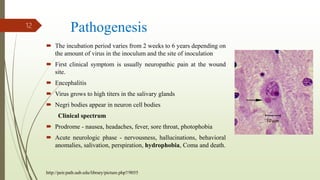
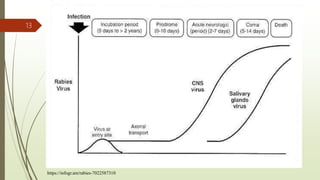
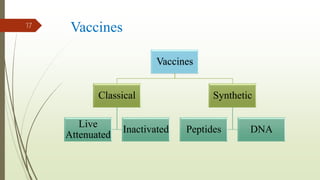
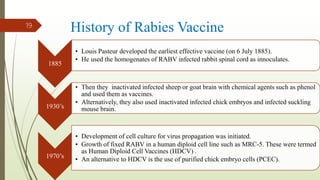
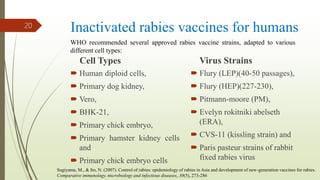
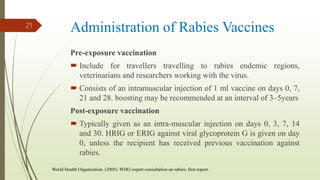
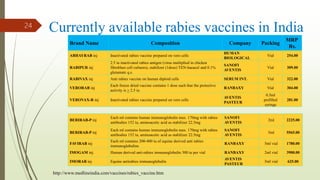
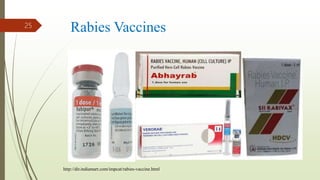
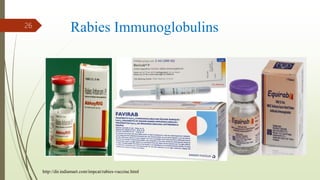
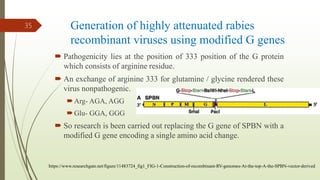
The document discusses rabies, a zoonotic viral disease, including its transmission, clinical symptoms, diagnosis, prevention, and vaccine development. It details the history of rabies vaccines since Louis Pasteur's first effective vaccine in 1885, presents current vaccine options and administration protocols, and outlines challenges such as vaccine efficacy and compliance. Additionally, it explores innovative vaccine research, including genetically modified virus vectors aimed at improving immunogenicity and safety.