Embed presentation
Downloaded 26 times



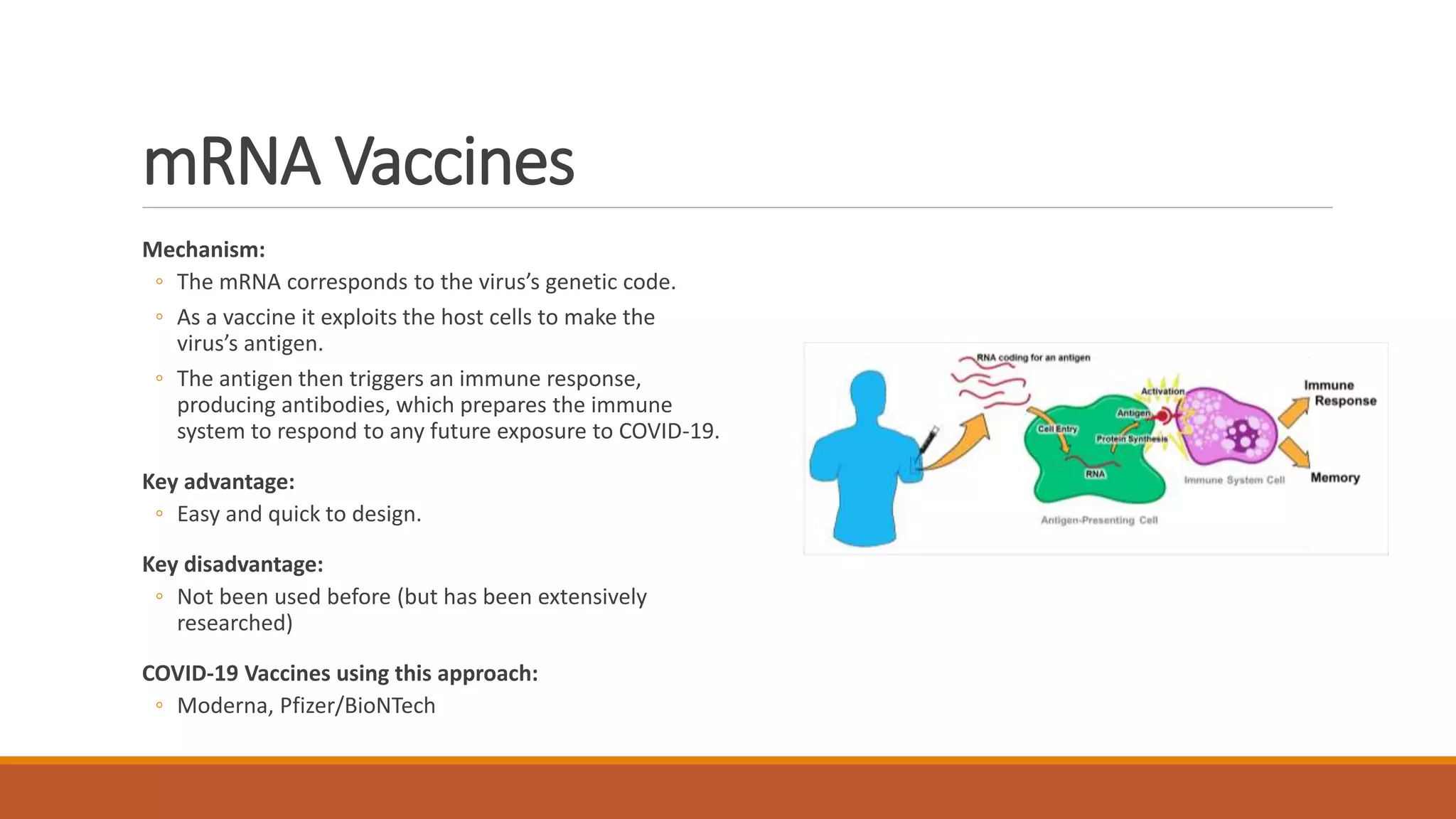
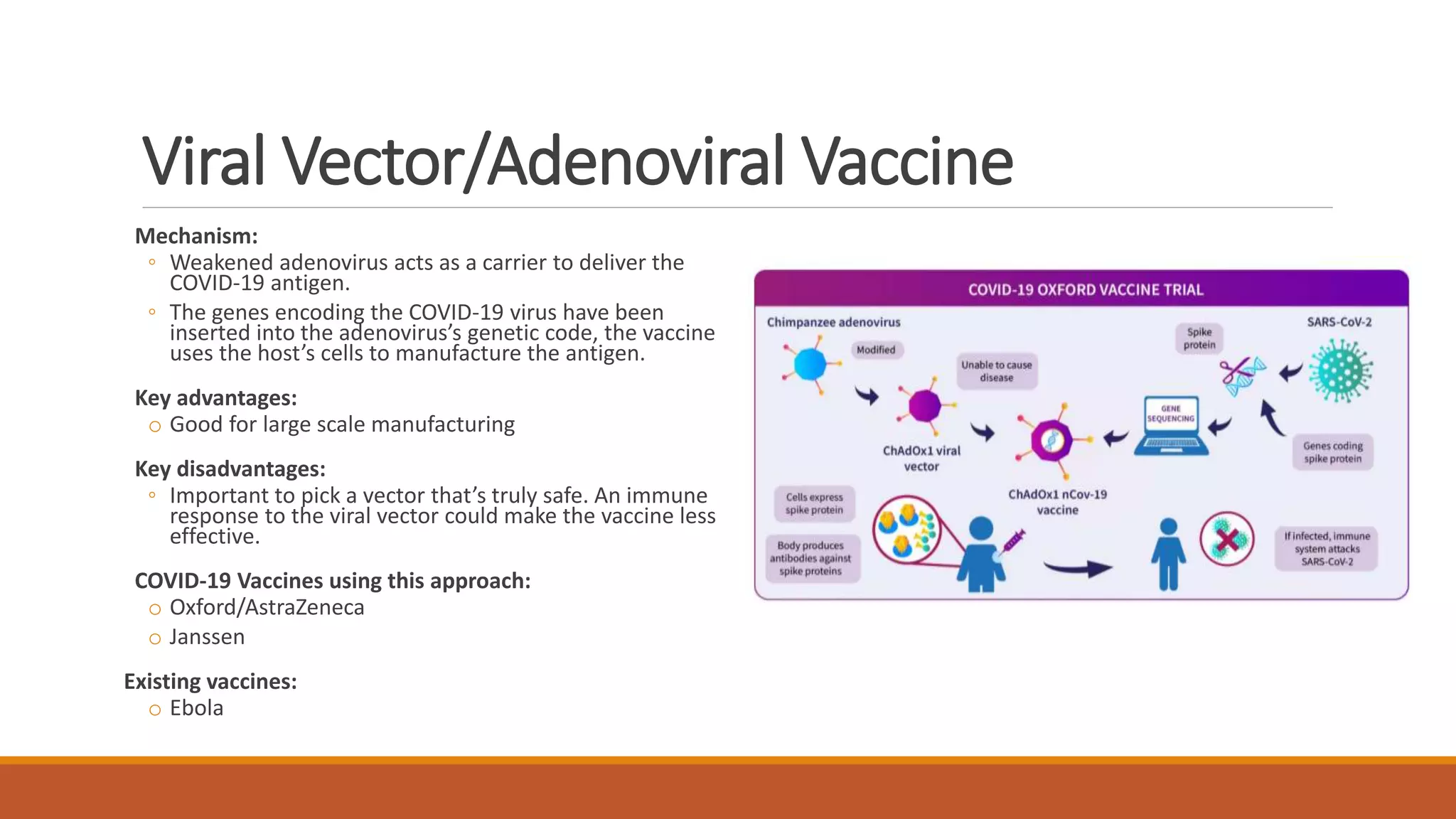

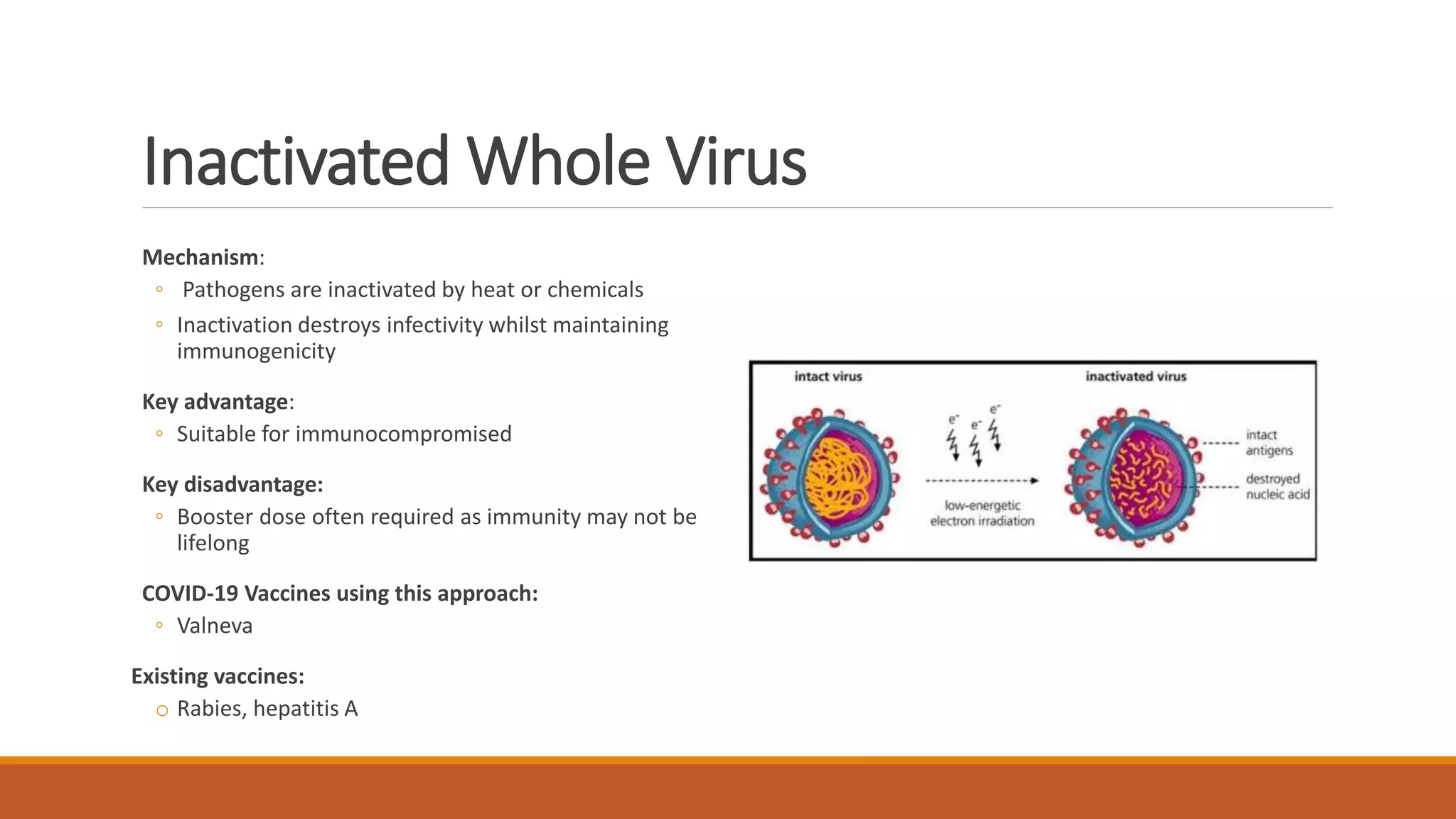
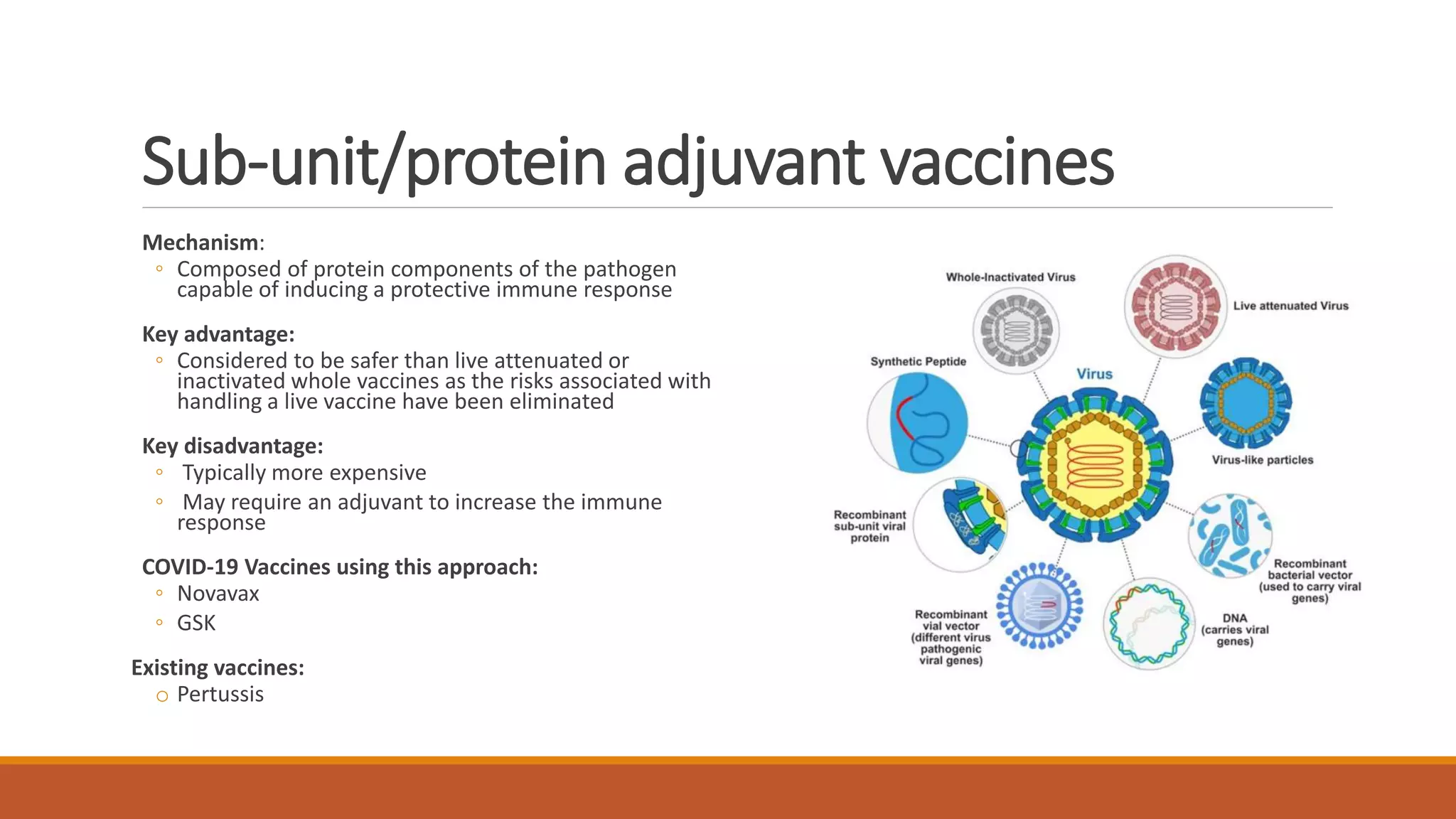










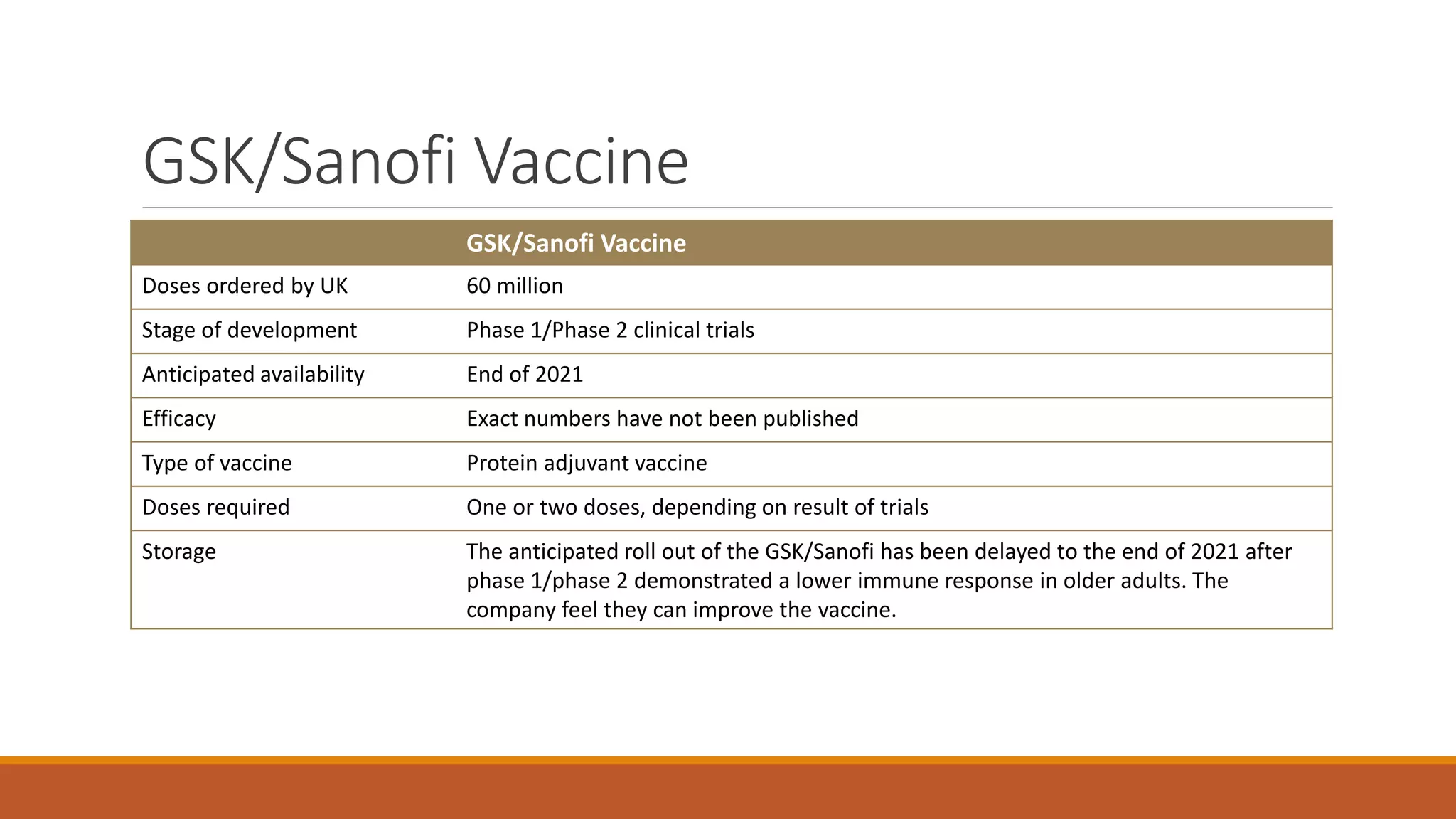




The document provides an overview of COVID-19 vaccines, detailing their clinical trial processes, types, and specific vaccines including Pfizer-BioNTech, Oxford-AstraZeneca, Moderna, Janssen, Novavax, GSK/Sanofi, and Valneva. It summarizes vaccine efficacy, storage requirements, and safety considerations, emphasizing the importance of ongoing monitoring post-approval. The document also highlights the extensive development of over 270 different COVID-19 vaccines globally and the UK's planned rollout of seven key vaccines.






















