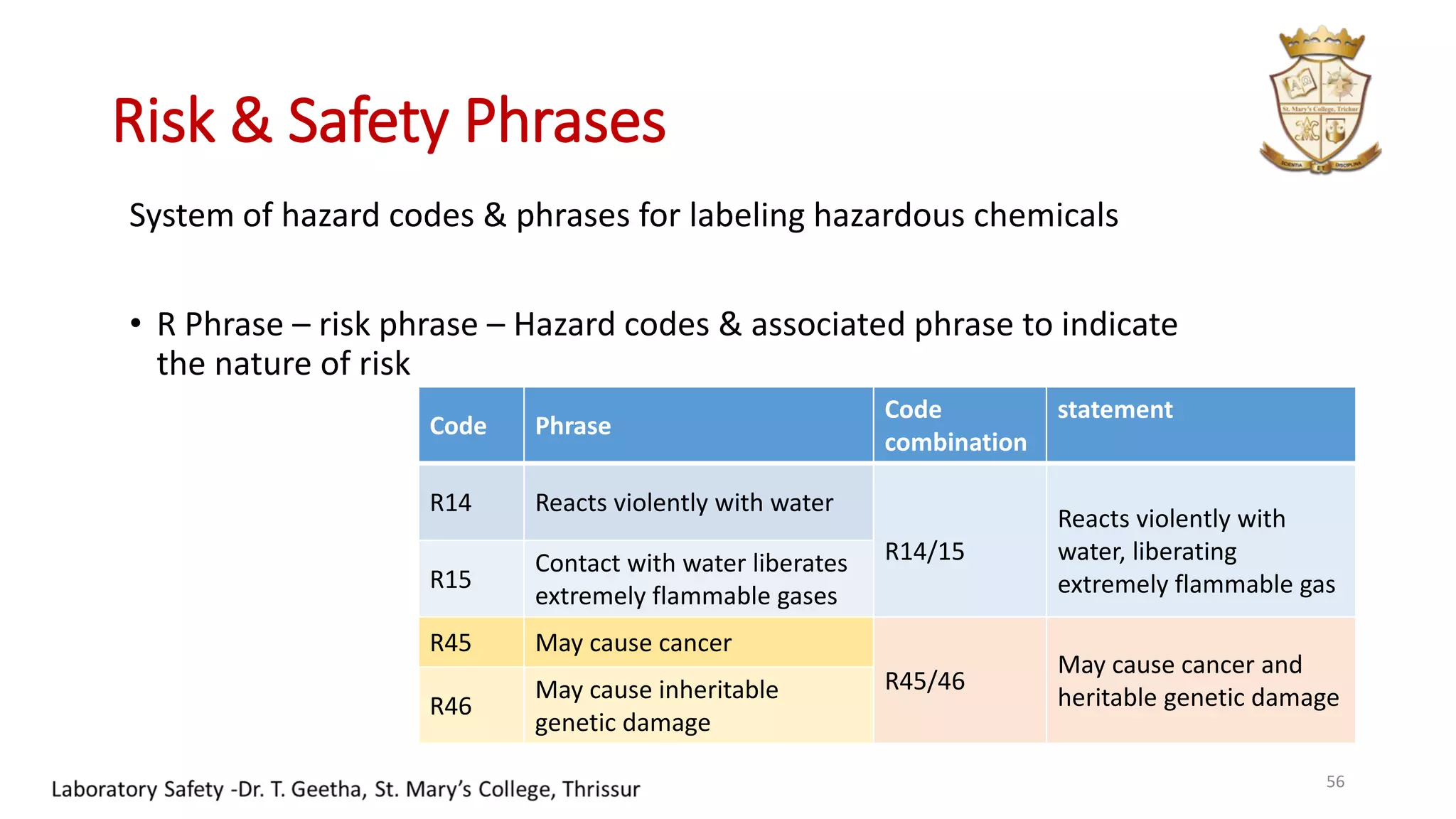
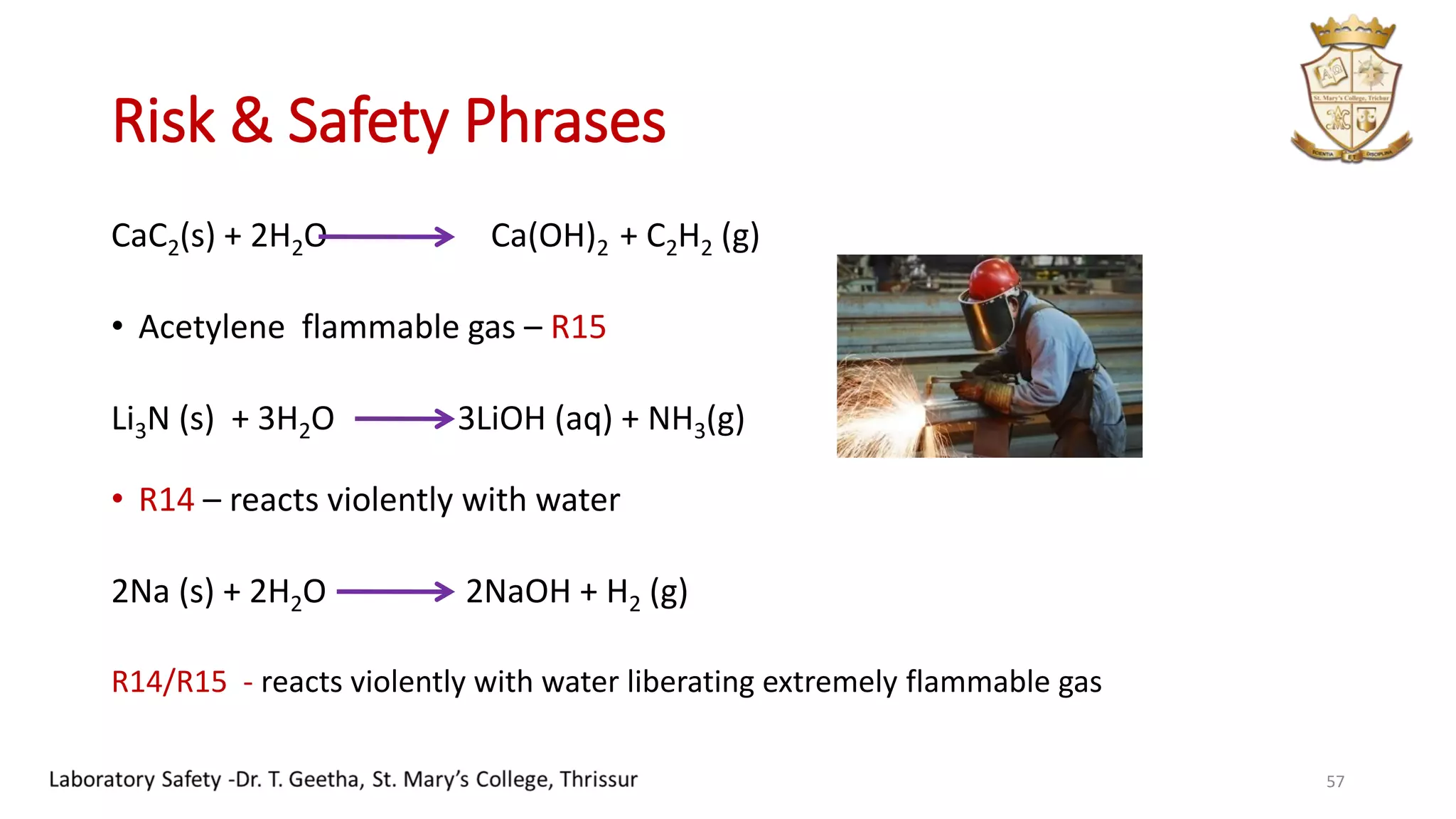
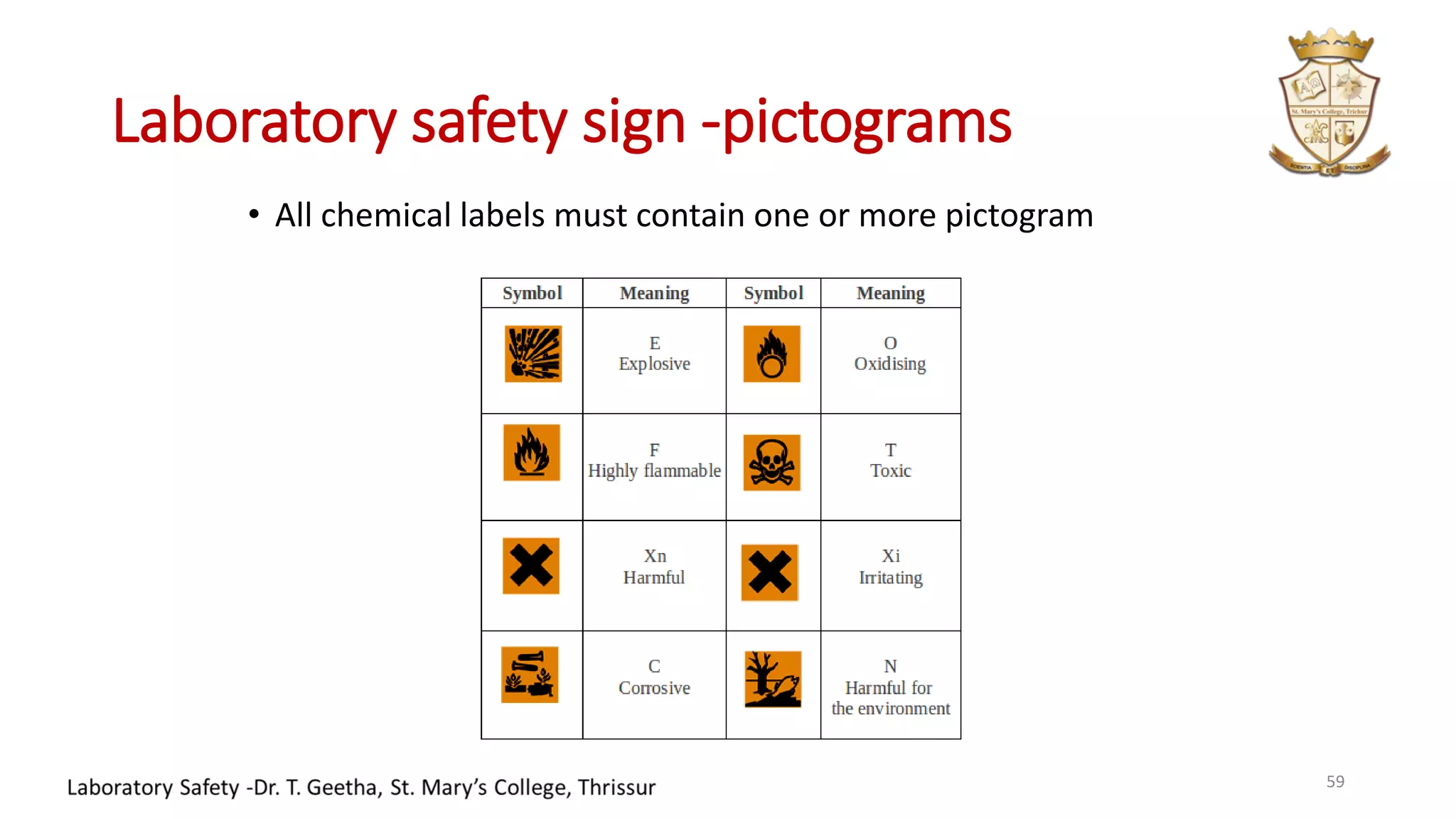
This document provides information on laboratory hygiene and safety. It discusses the importance of safety in laboratories and identifies various hazards like chemicals. It outlines factors that contribute to safety like environmental, personal and behavioral factors. The document provides detailed safety rules for laboratories and handling of chemicals. It describes storage and disposal procedures. Finally, it discusses important lessons from an incident of mercury contamination at a thermometer factory in Kodaikanal, India.