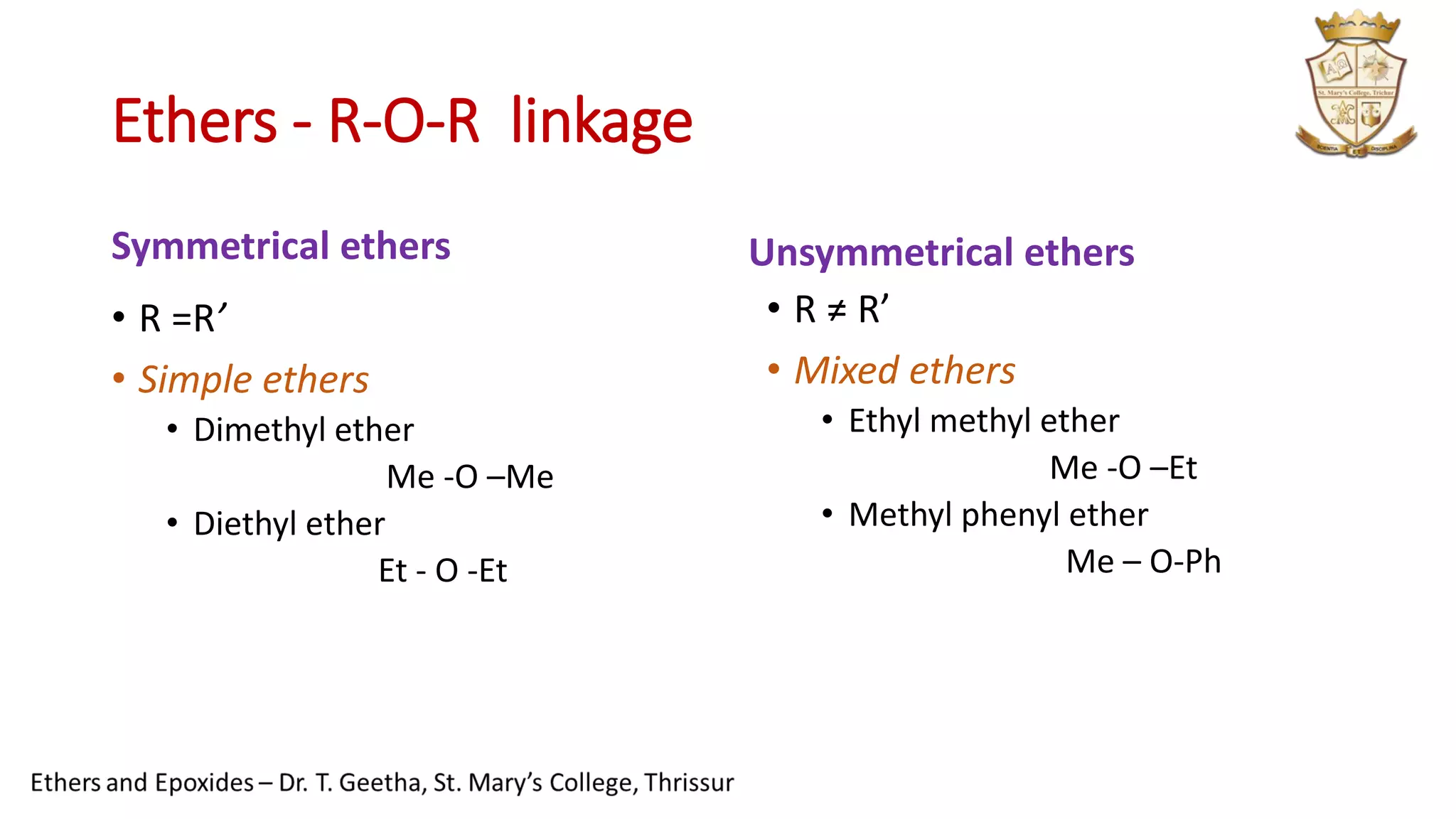
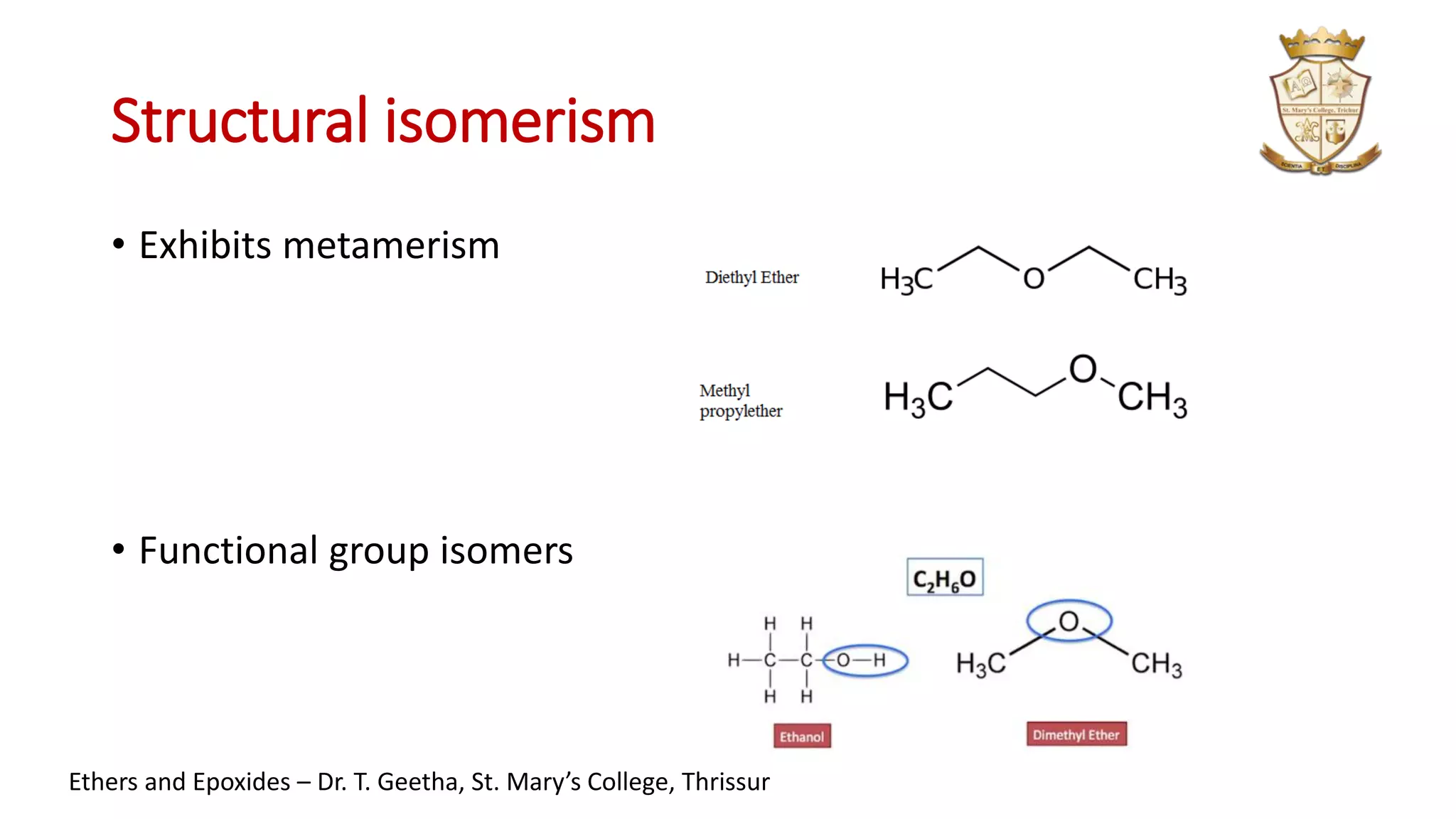
1. Ethers have the general structure R-O-R' and are classified as symmetrical or unsymmetrical. Common ethers include diethyl ether and anisole.
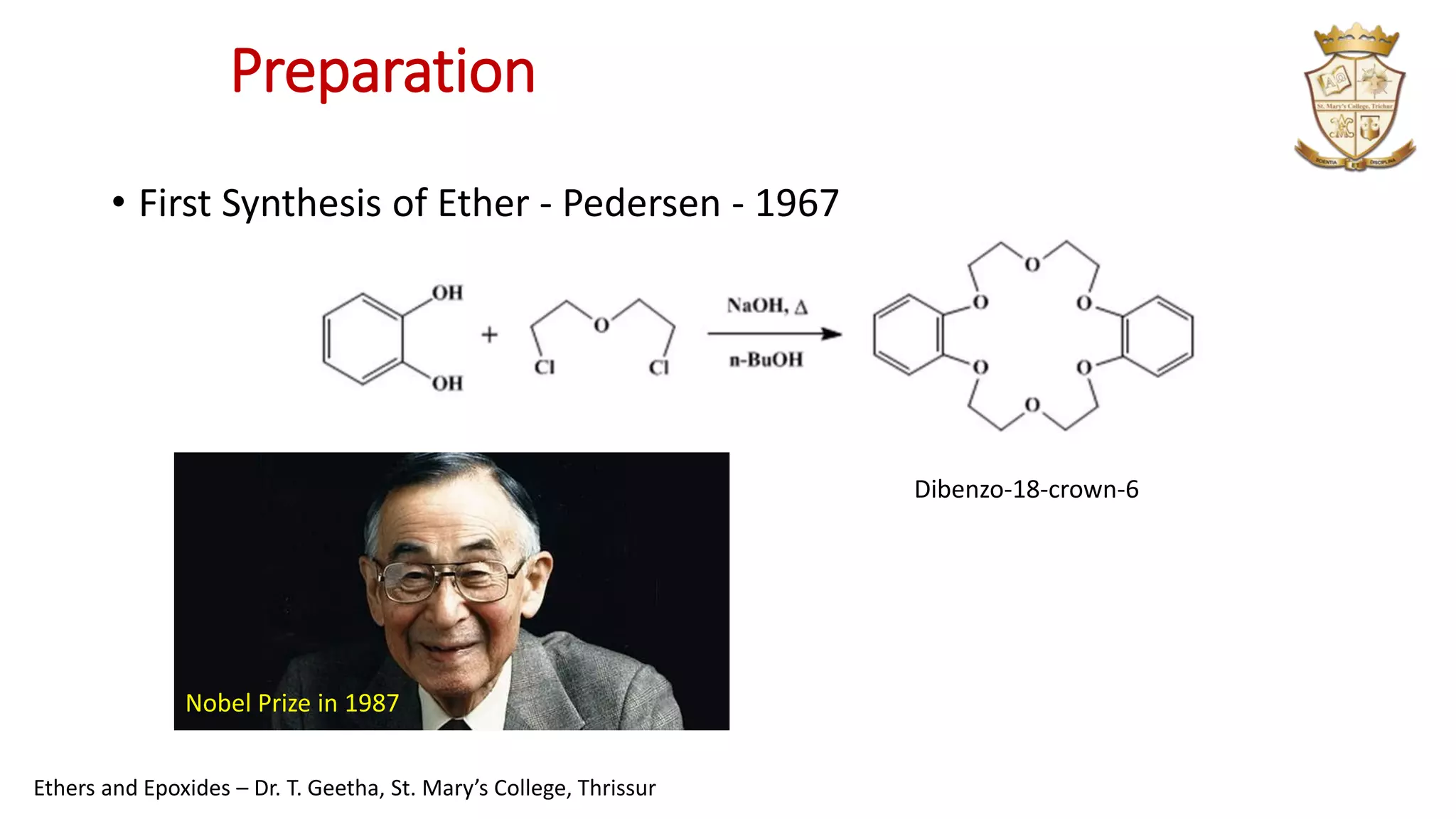
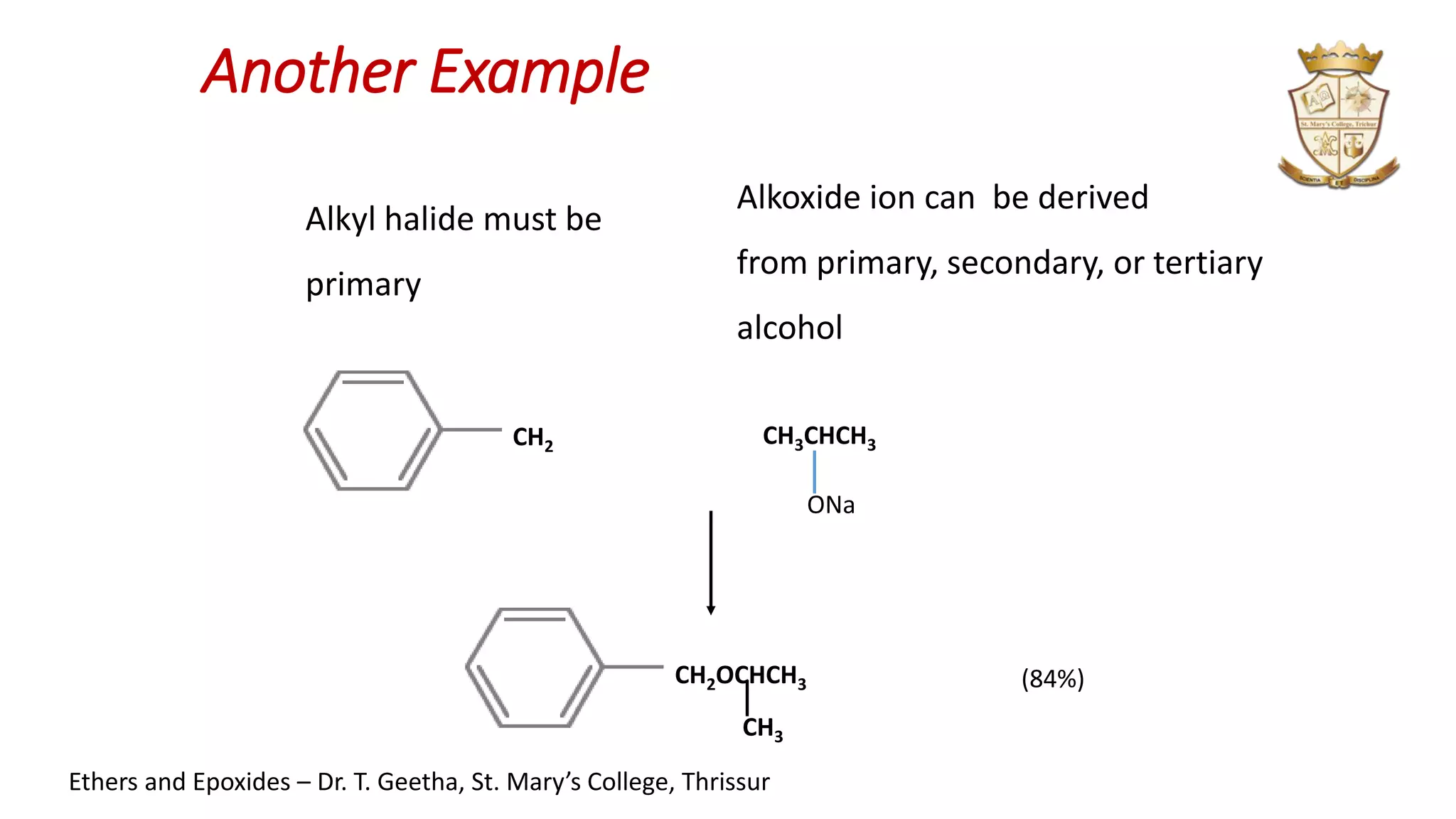
2. Ethers can be synthesized via Williamson ether synthesis using sodium alkoxides and alkyl halides or via acid-catalyzed dehydration of alcohols.
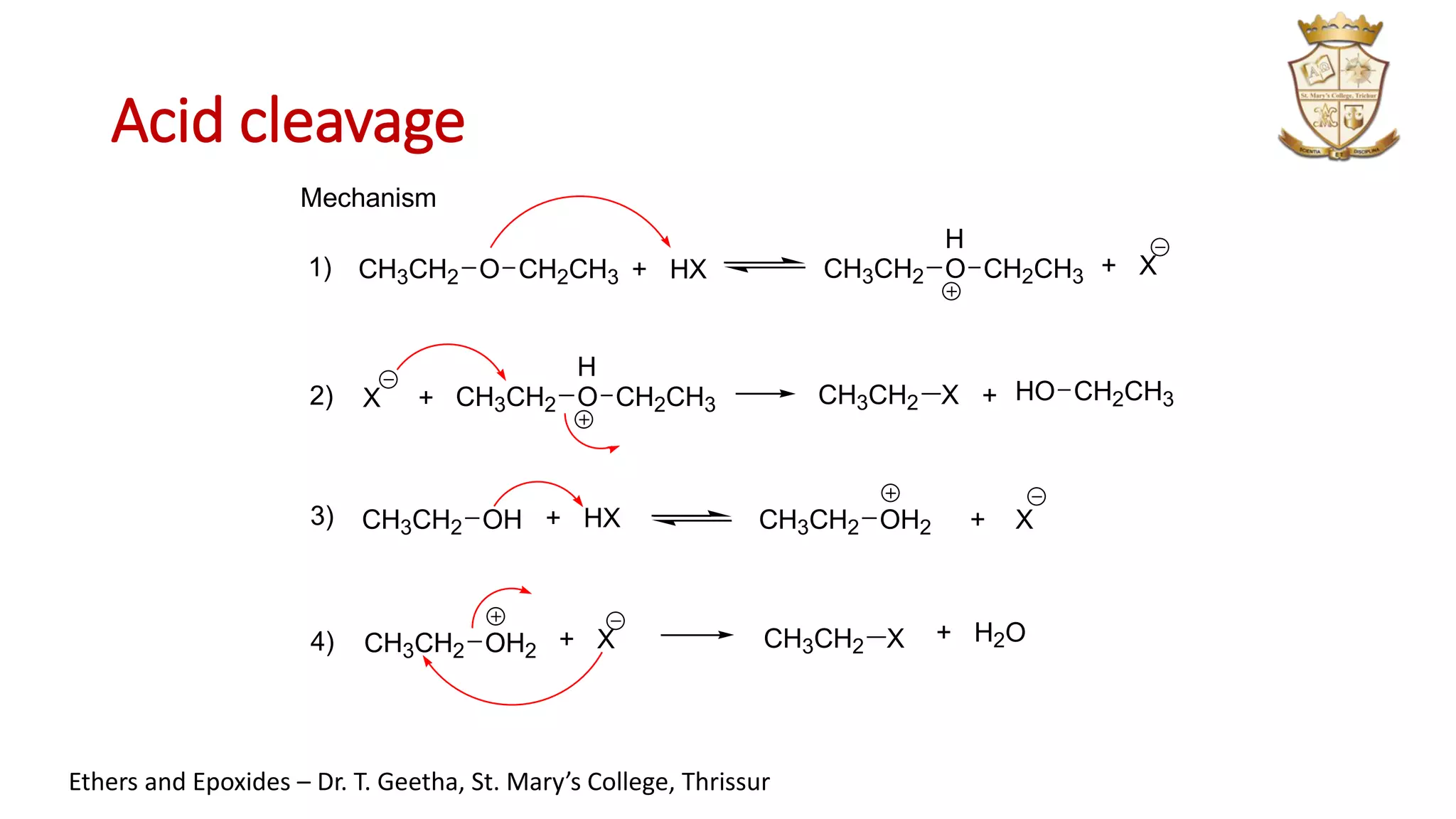
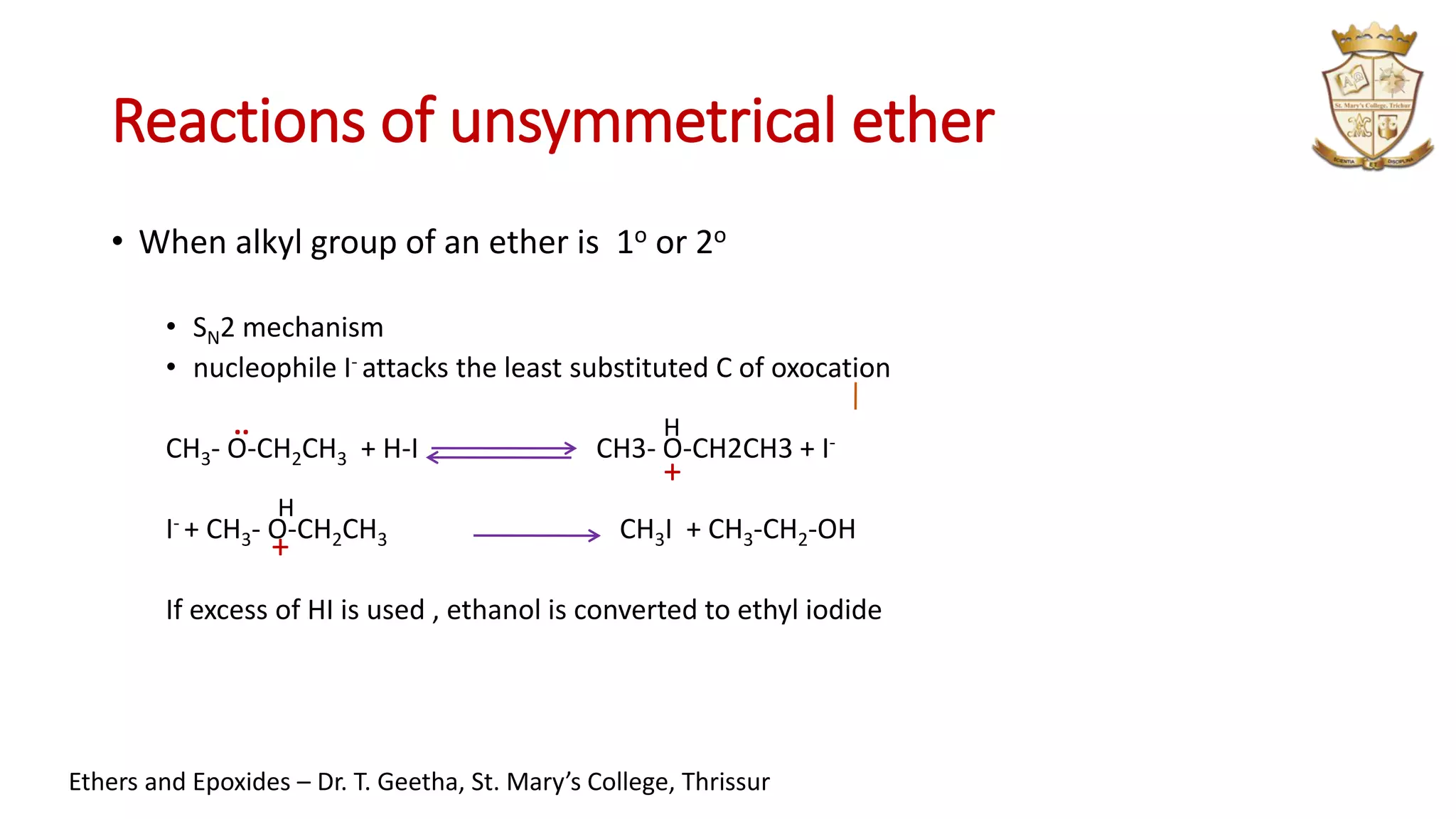
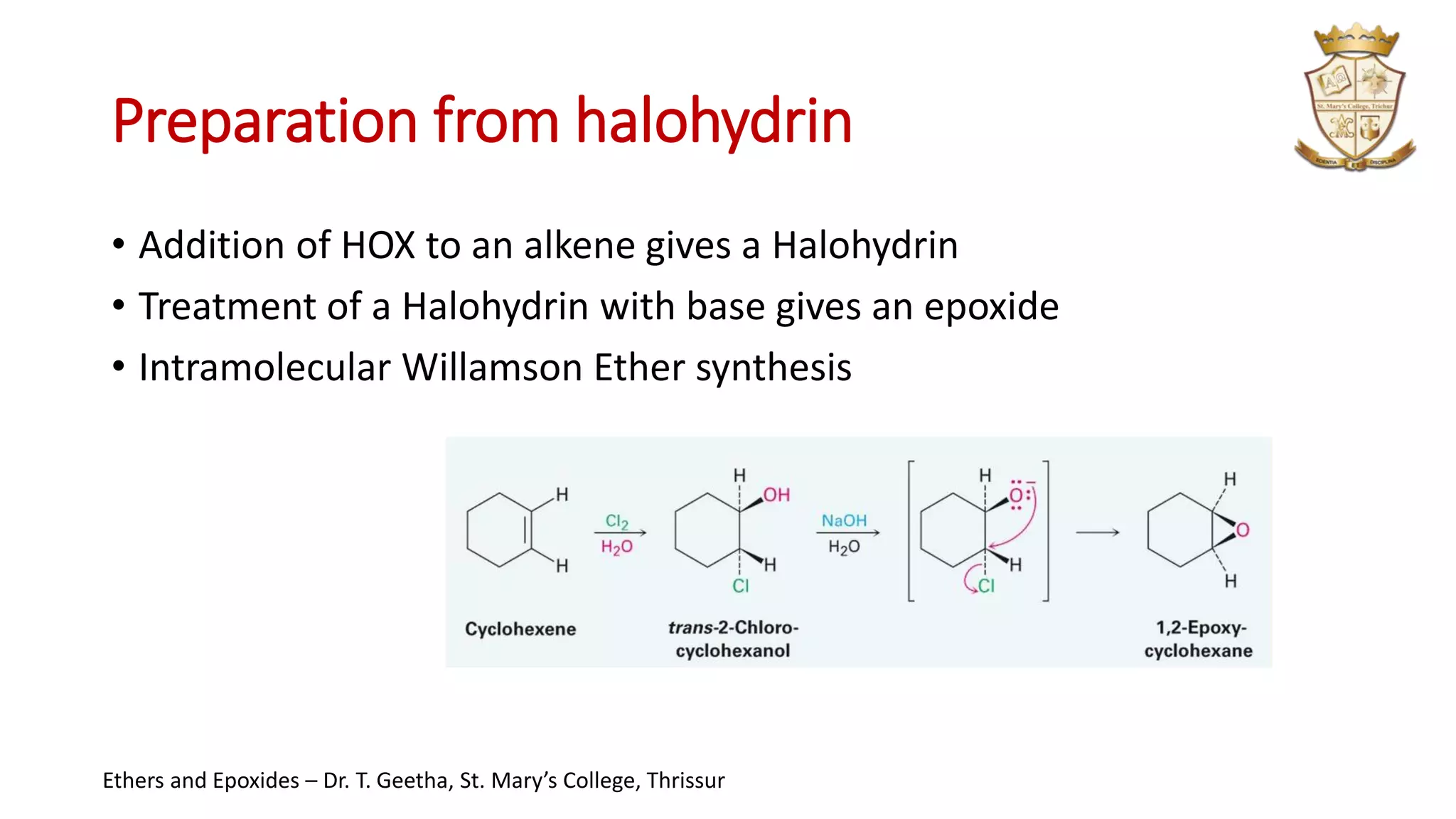
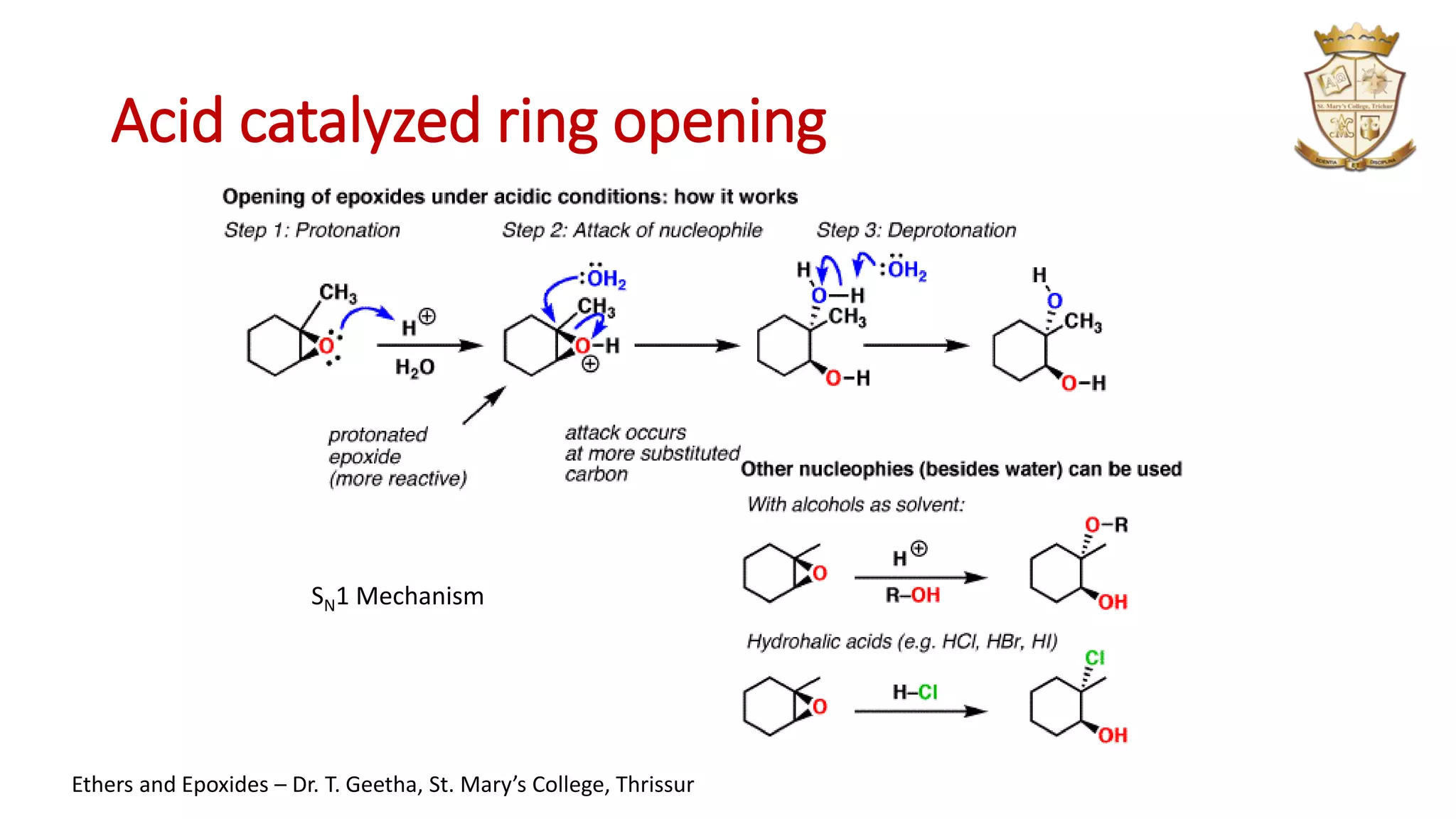
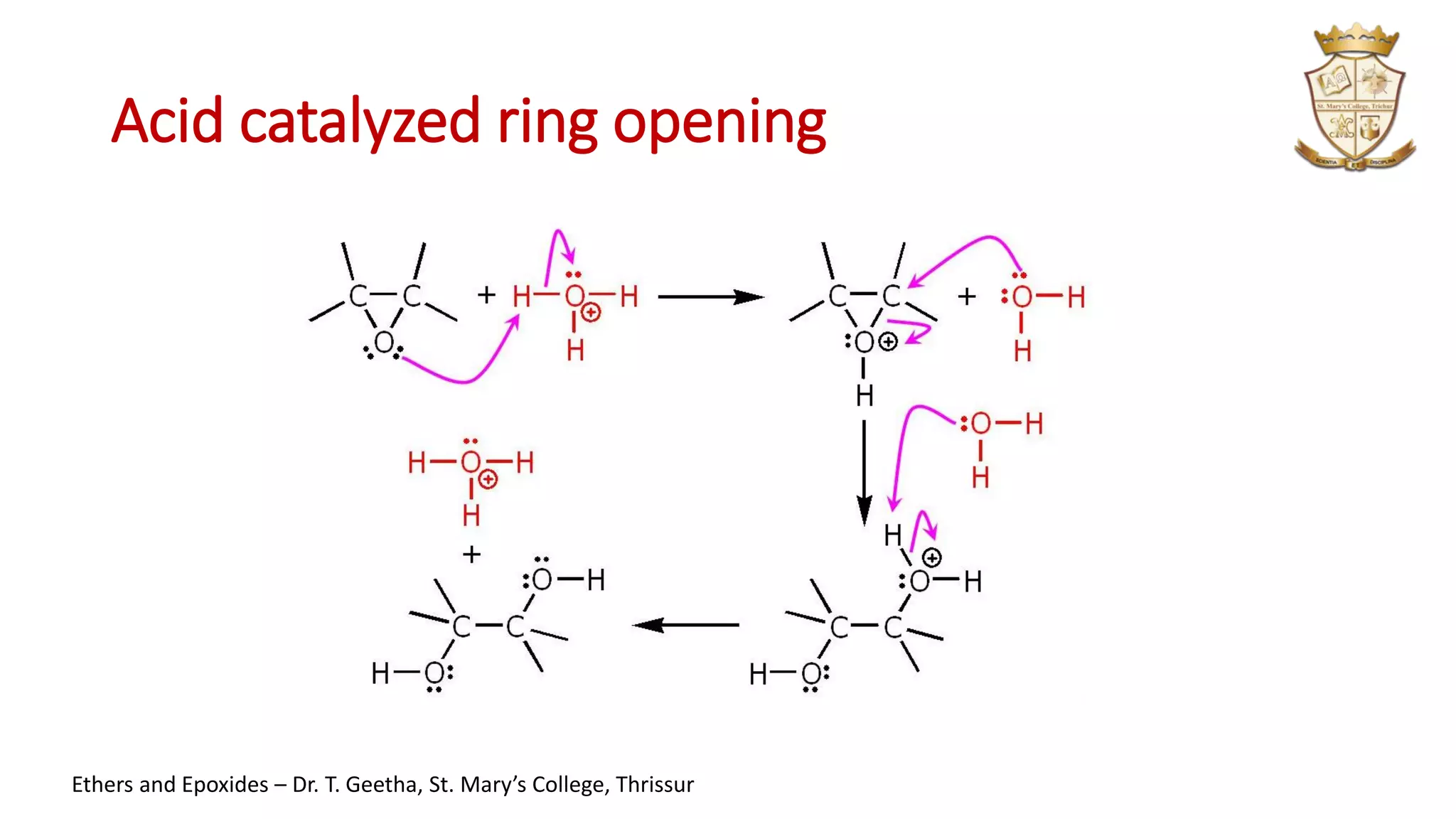
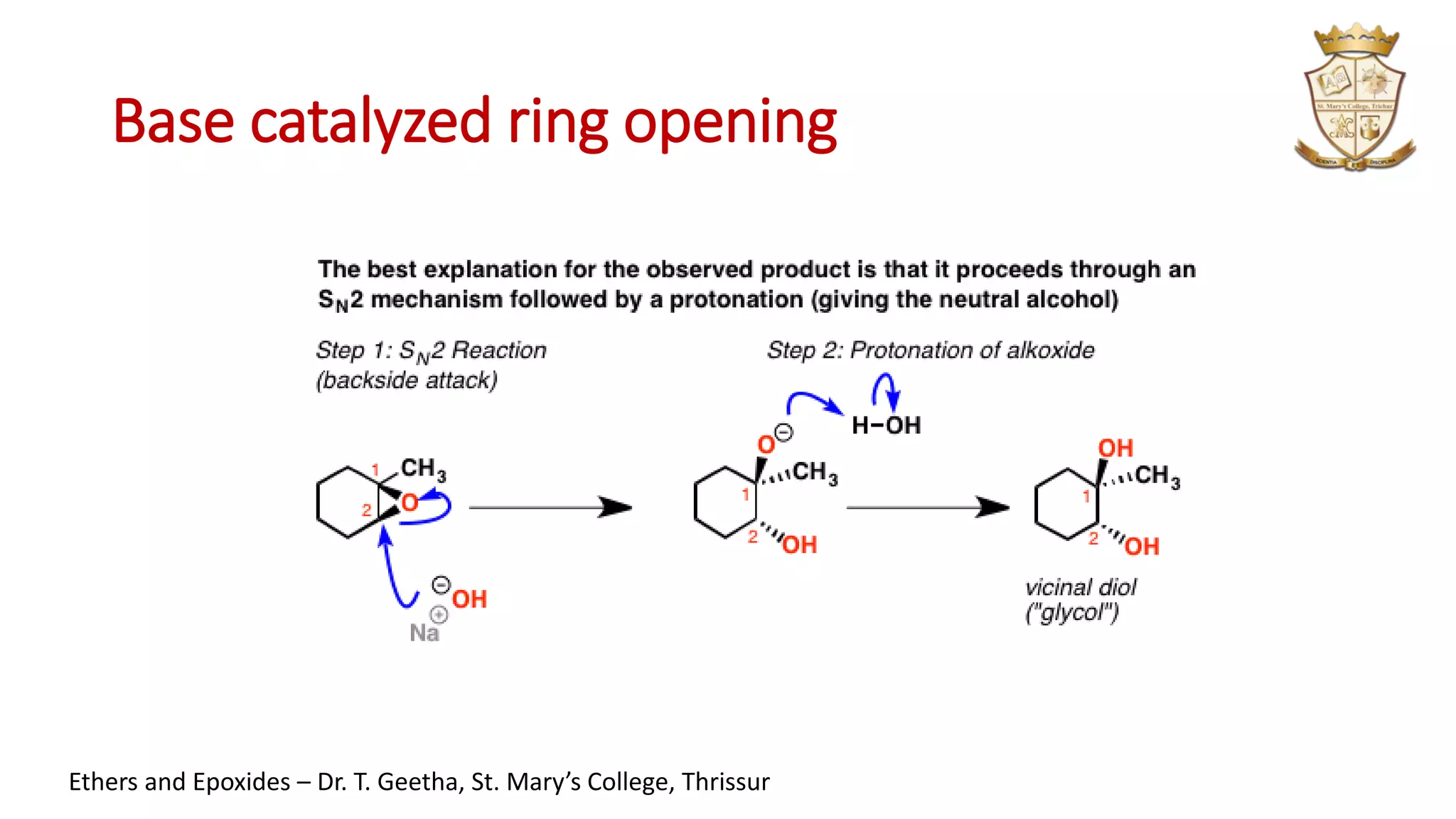
3. Epoxides are cyclic ethers with a 3-membered ring. They react with acids and bases via SN1 and SN2 mechanisms respectively to open the ring.



















![Claisen rearrangement
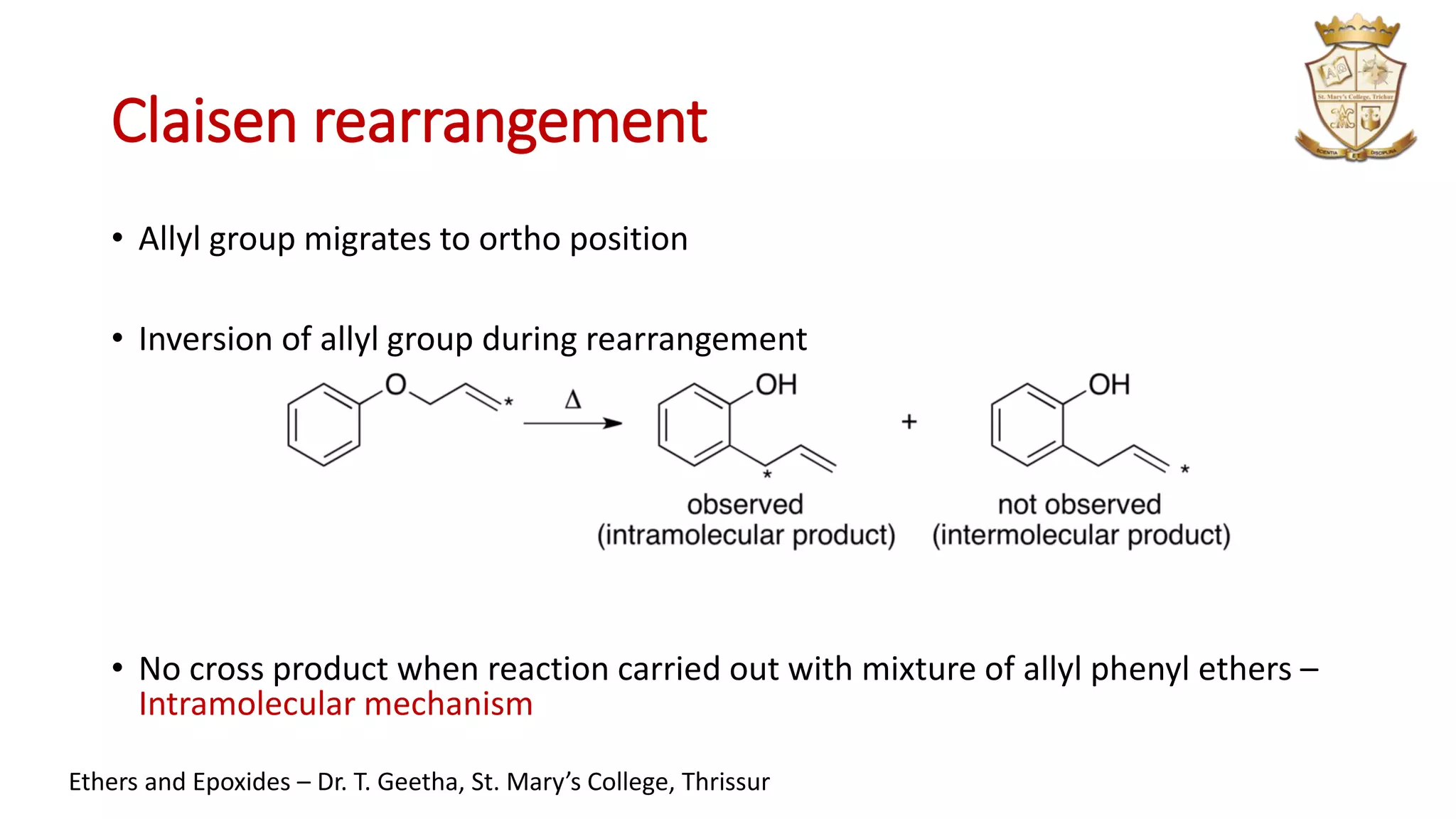
• [3,3]-sigmatropic rearrangement
• allyl phenyl ether is converted thermally to an unsaturated carbonyl compound
followed by rearomatisation
Ethers and Epoxides – Dr. T. Geetha, St. Mary’s College, Thrissur](https://image.slidesharecdn.com/ethersandepoxide-190926174338/75/Ethers-and-epoxide-25-2048.jpg)








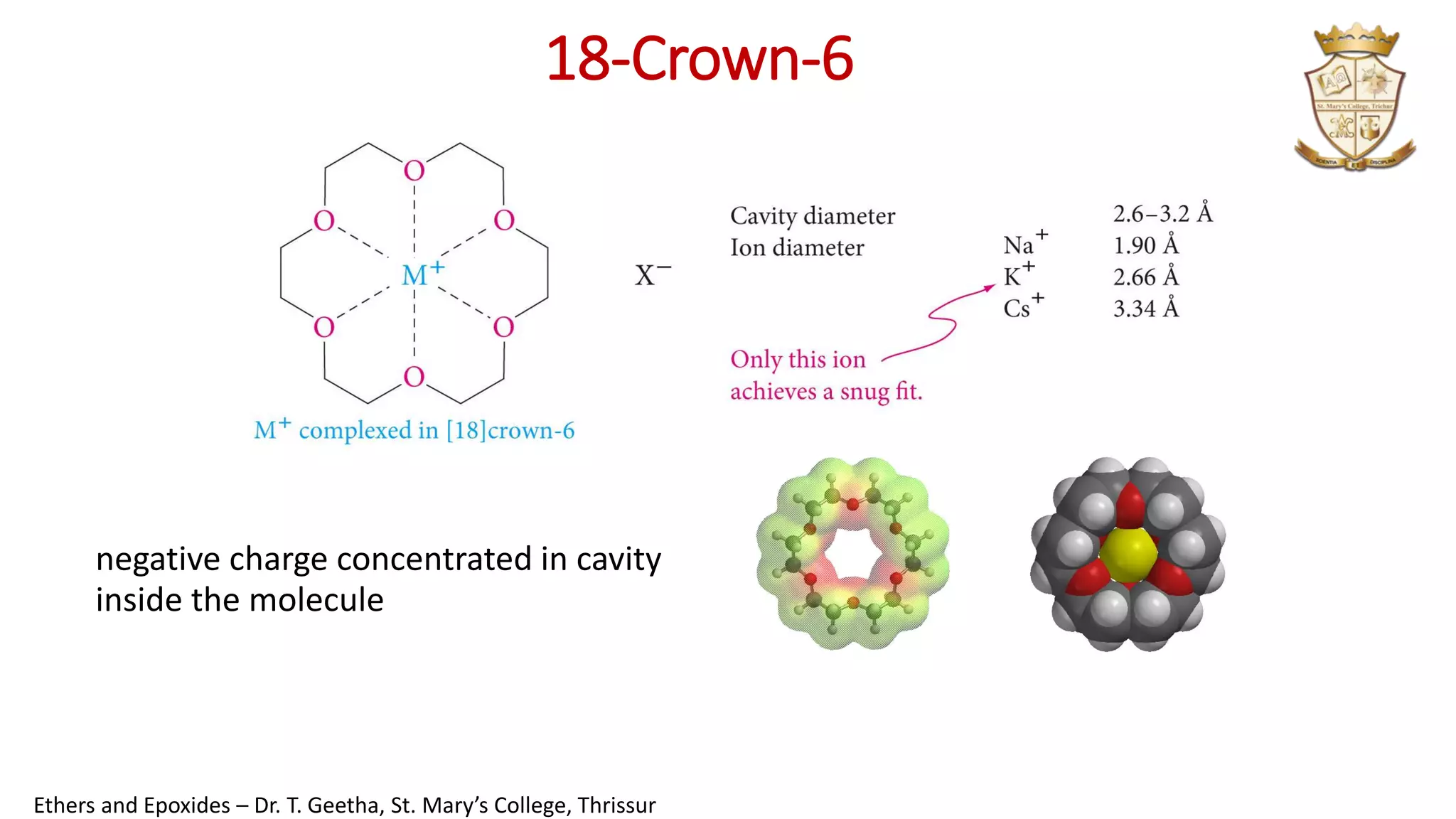
![Crown Ether
•Structure
cyclic polyethers derived from repeating
—OCH2CH2— units
•Properties
form stable complexes with metal ions
•Applications
synthetic reactions involving anions
•Naming
x = total no. of atoms in ring: [x] Crown- no. of oxygen atoms
Ethers and Epoxides – Dr. T. Geetha, St. Mary’s College, Thrissur](https://image.slidesharecdn.com/ethersandepoxide-190926174338/75/Ethers-and-epoxide-39-2048.jpg)