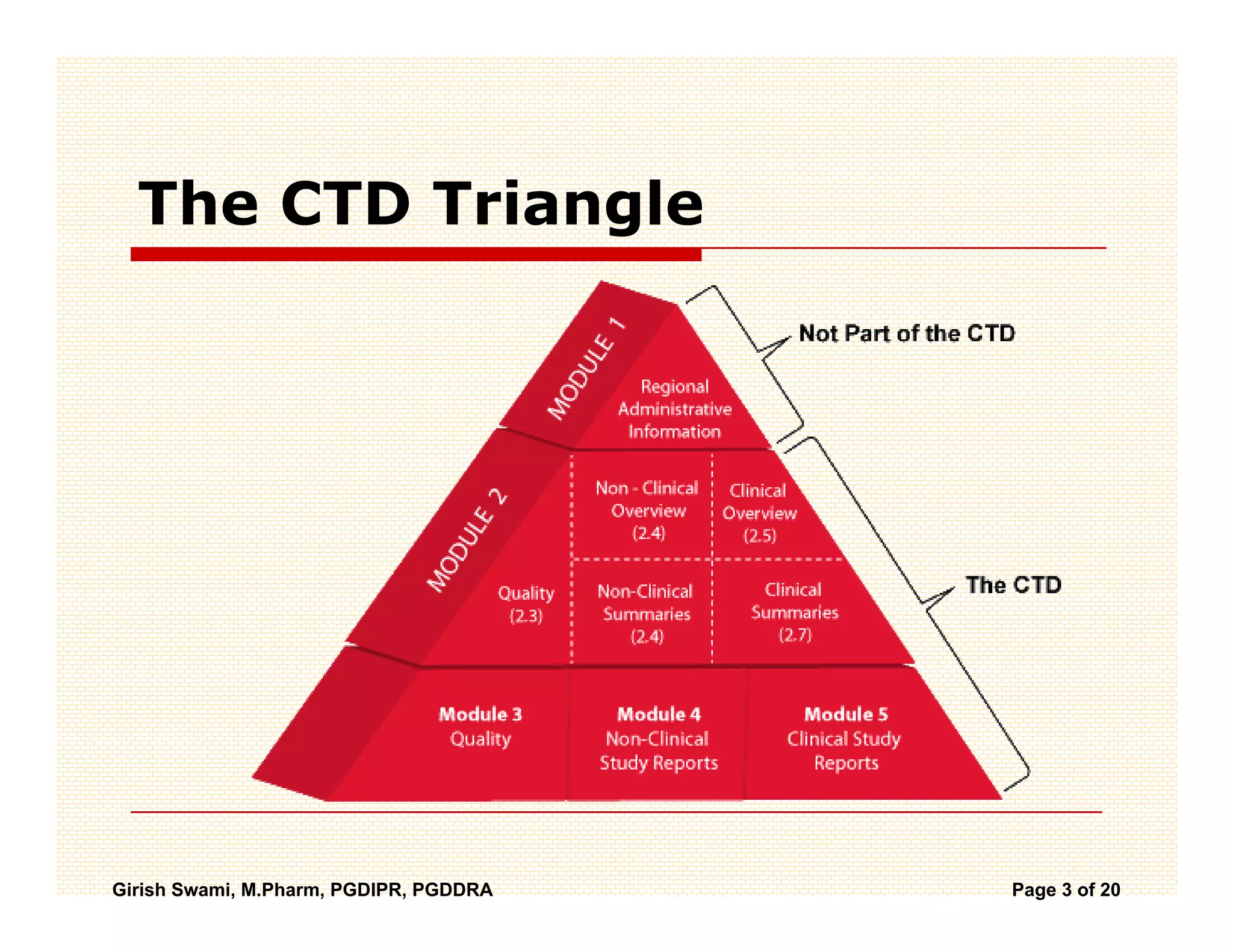
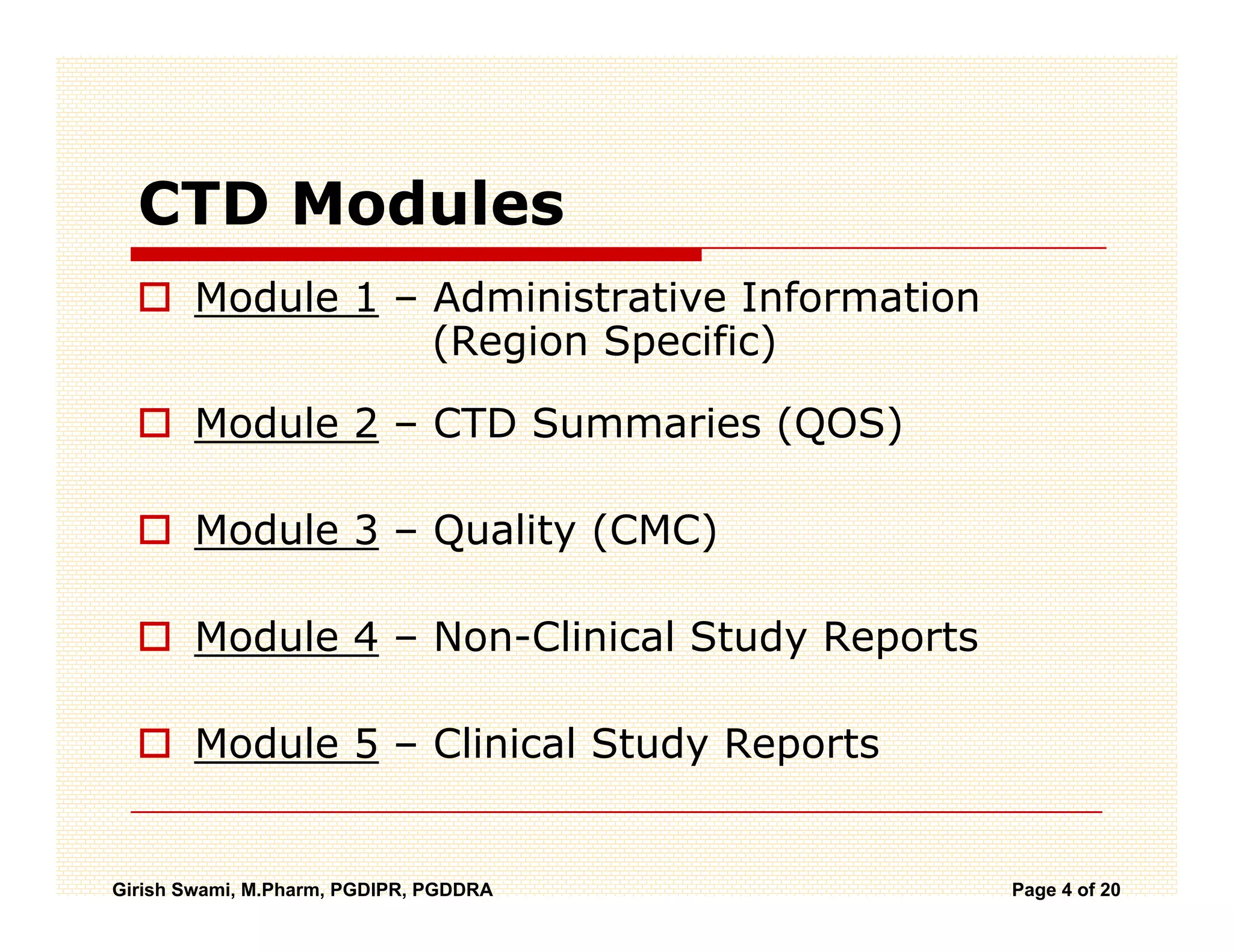
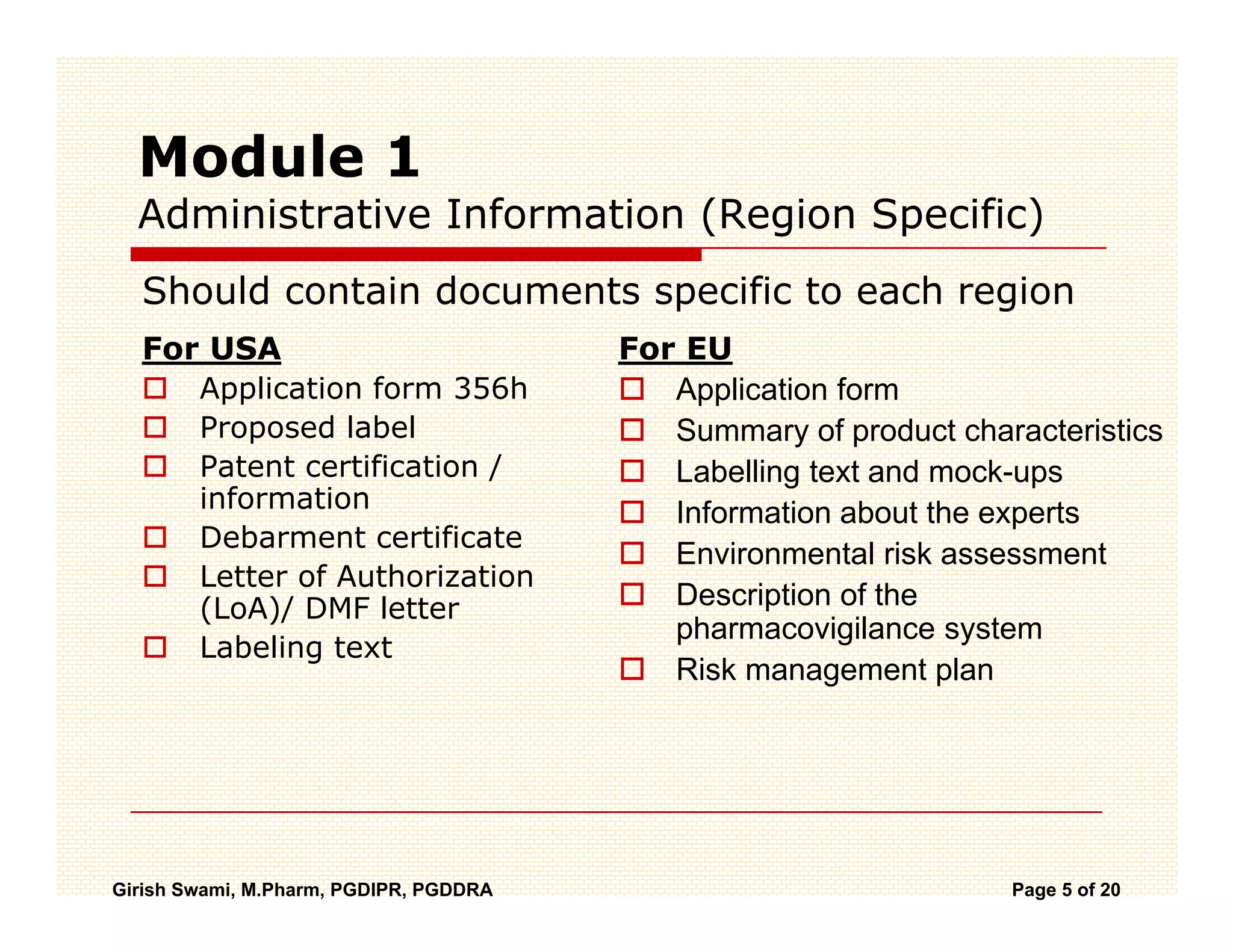
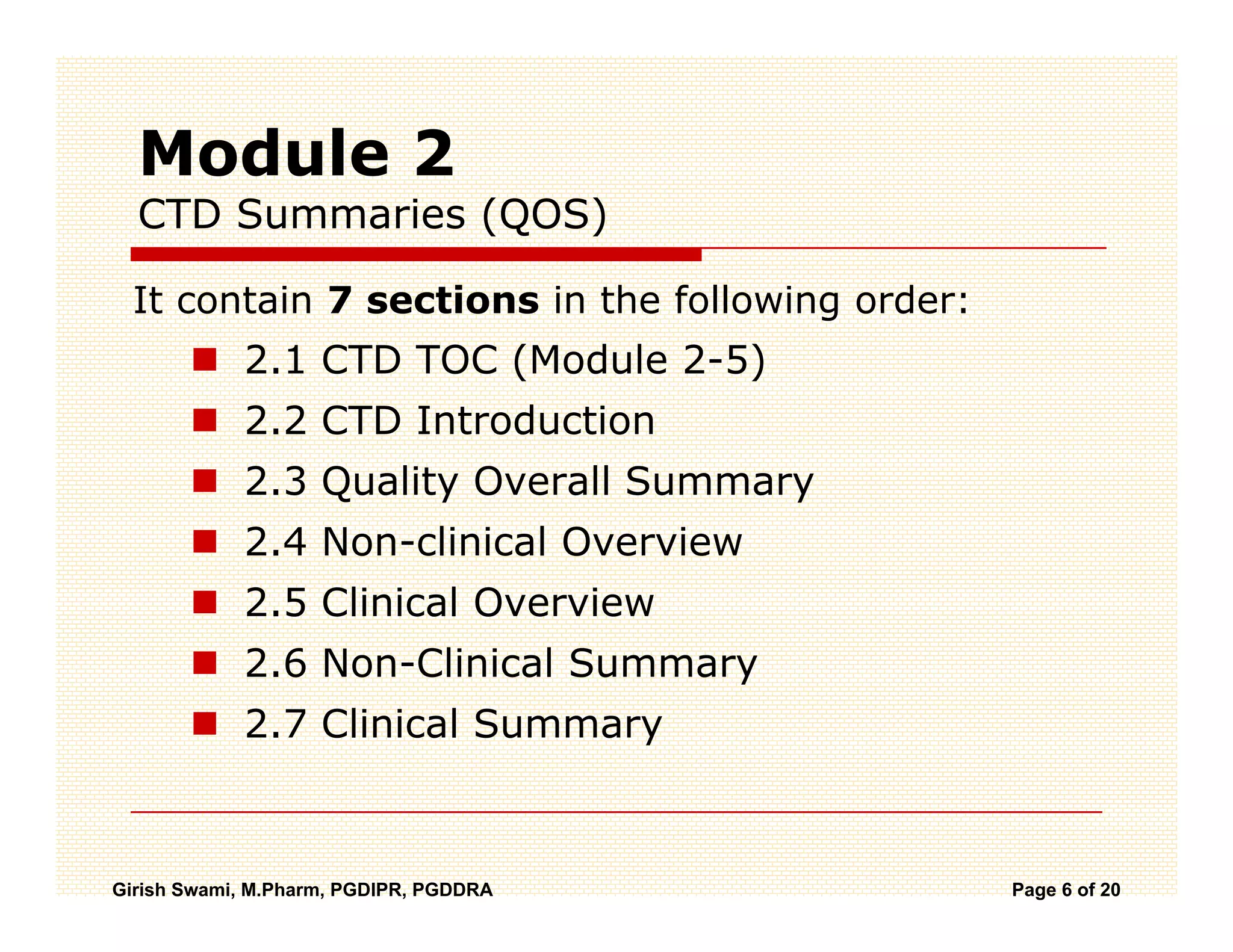
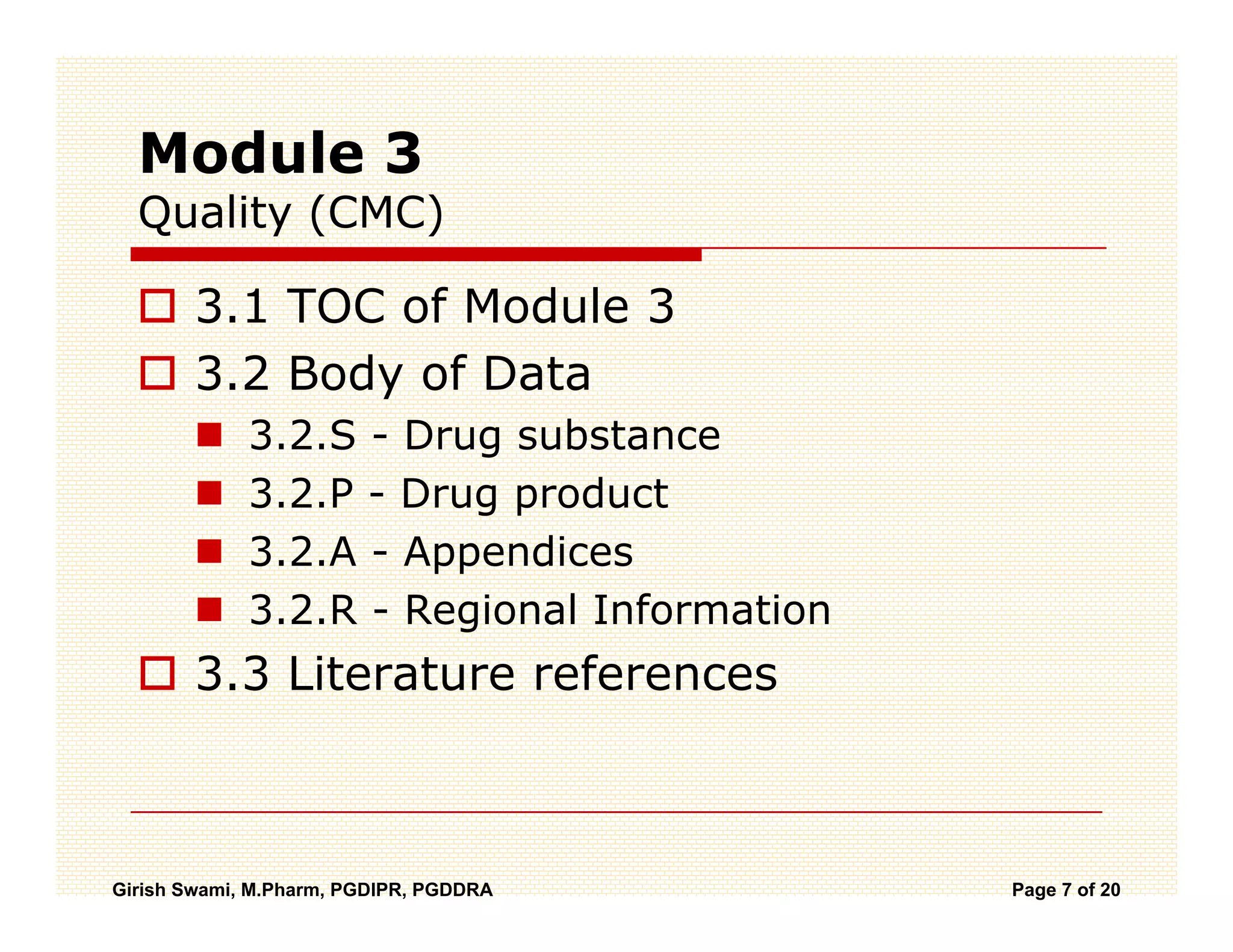
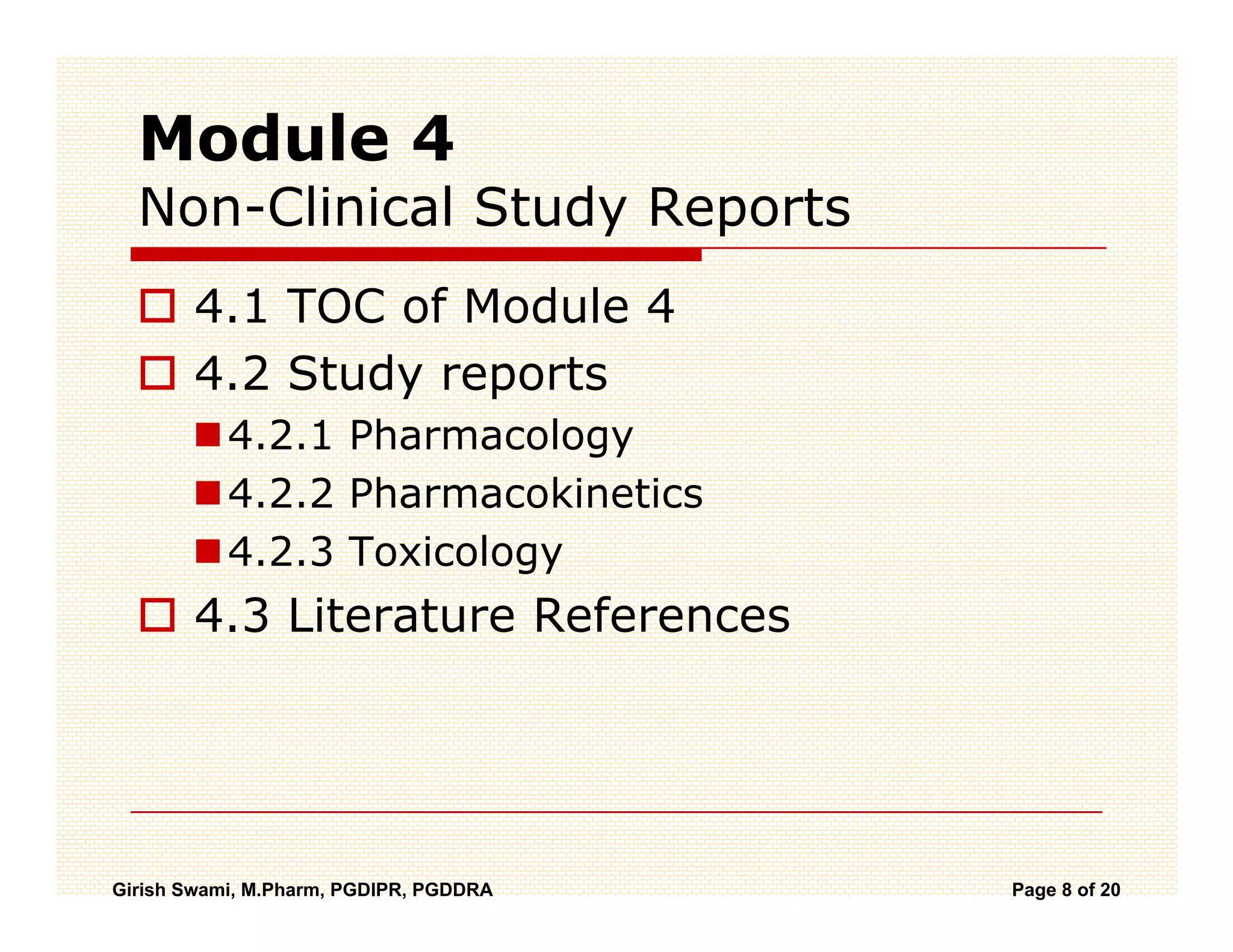
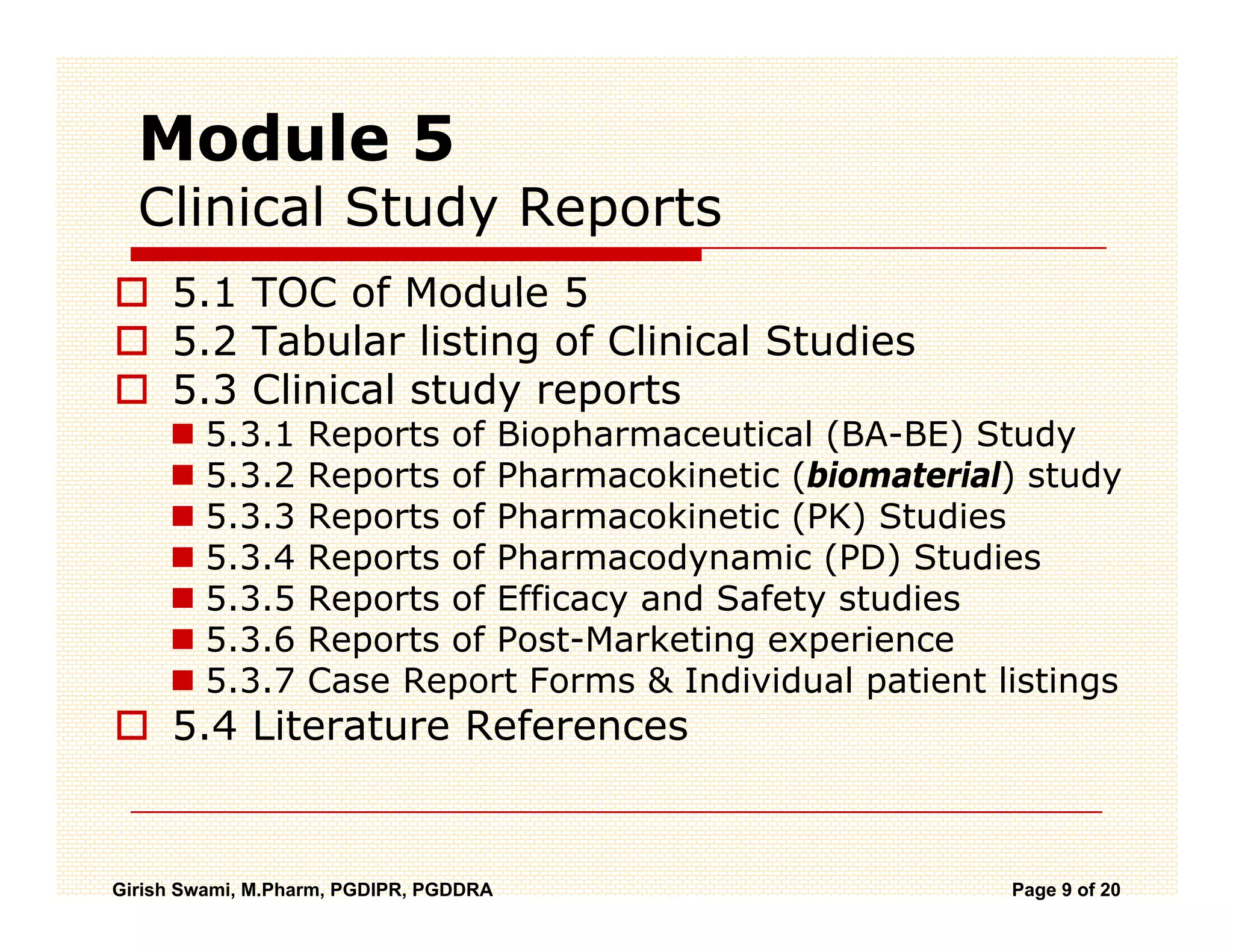
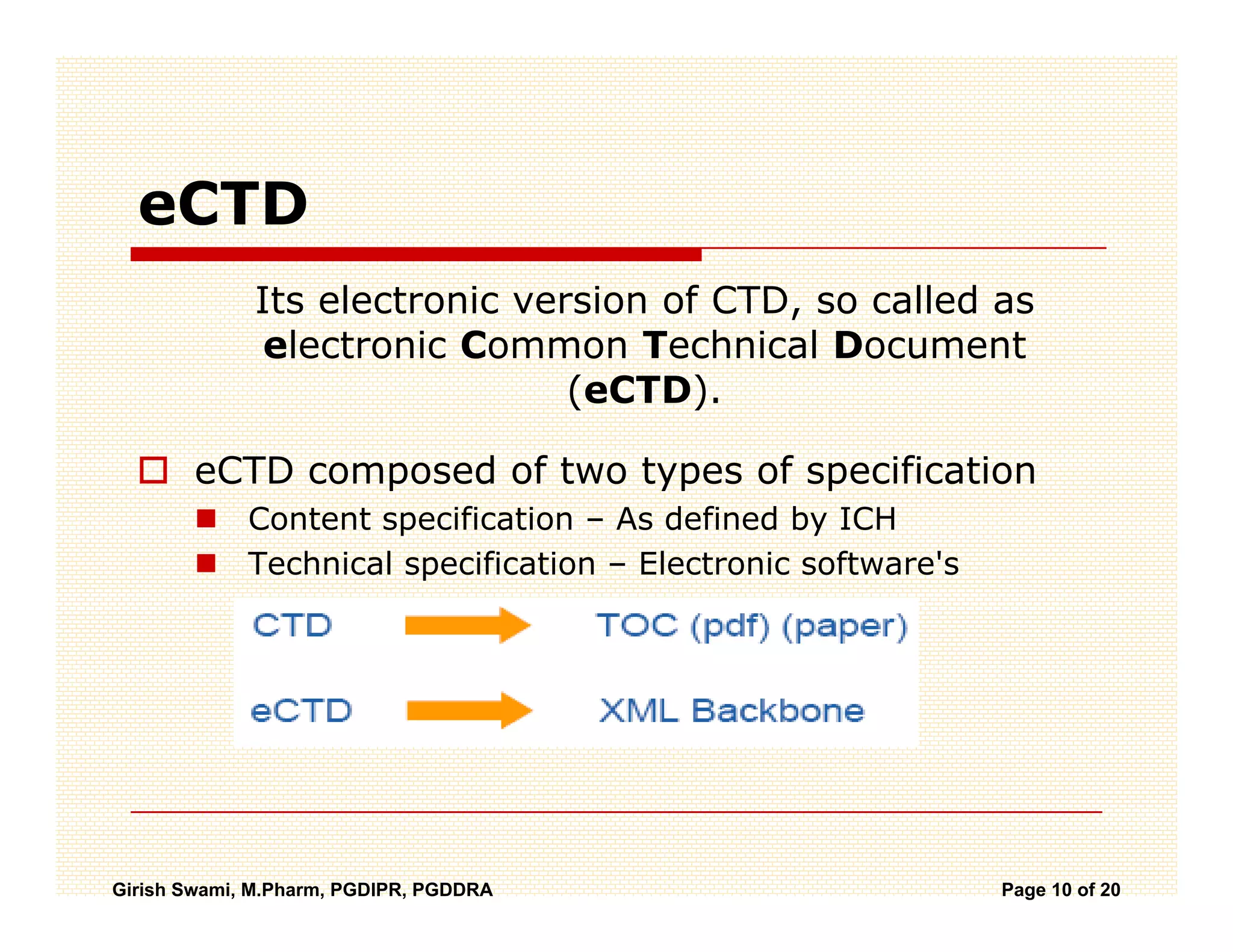
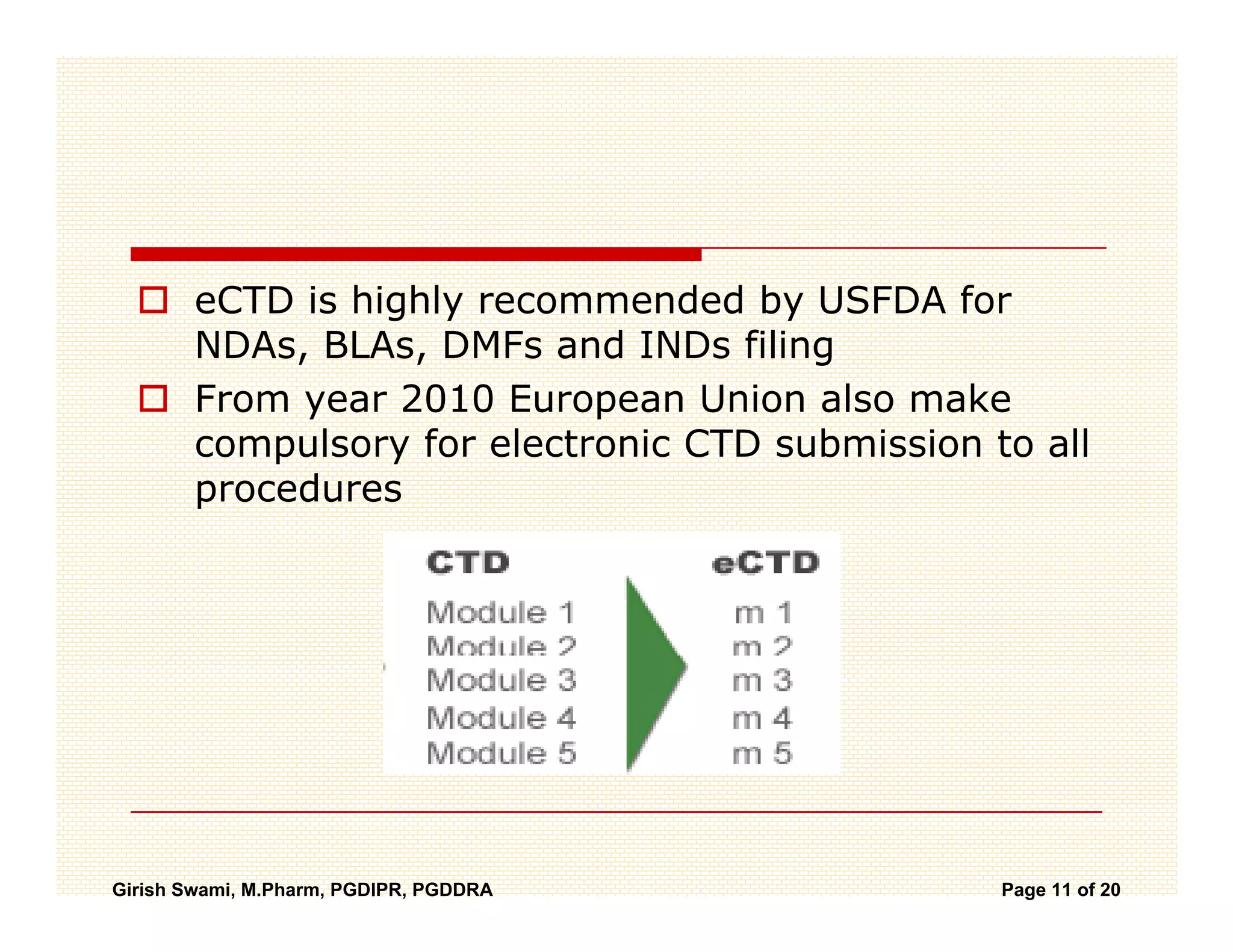
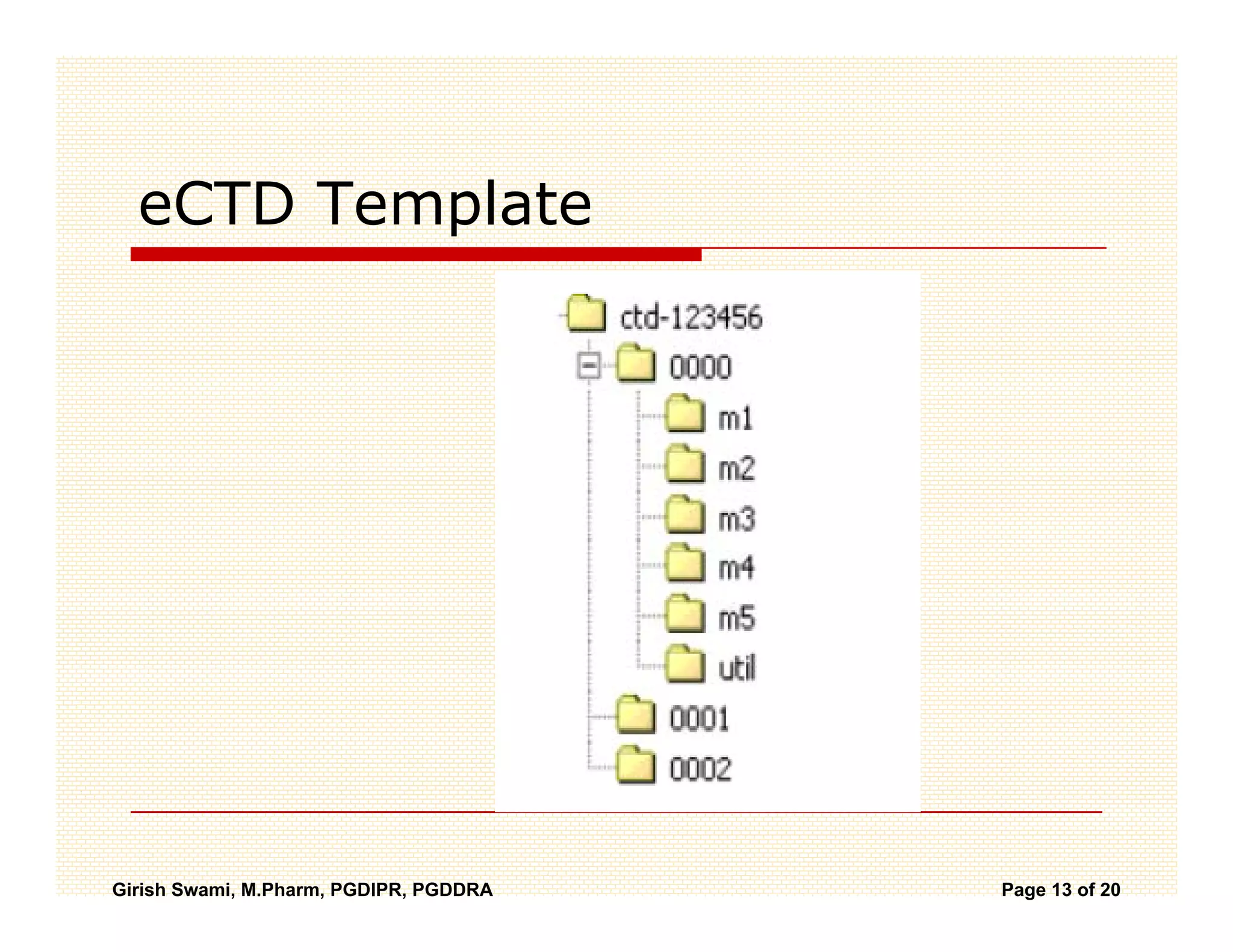
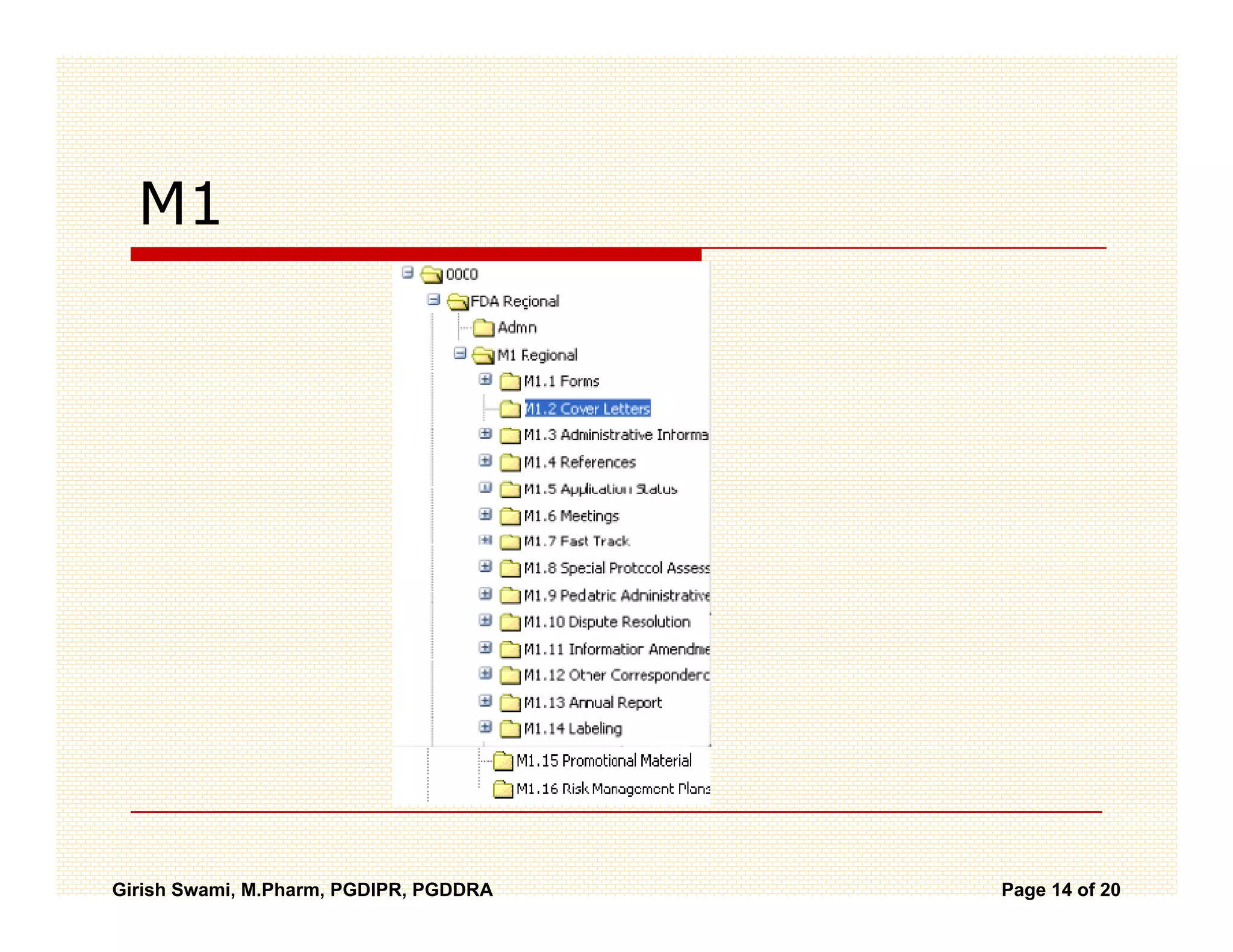
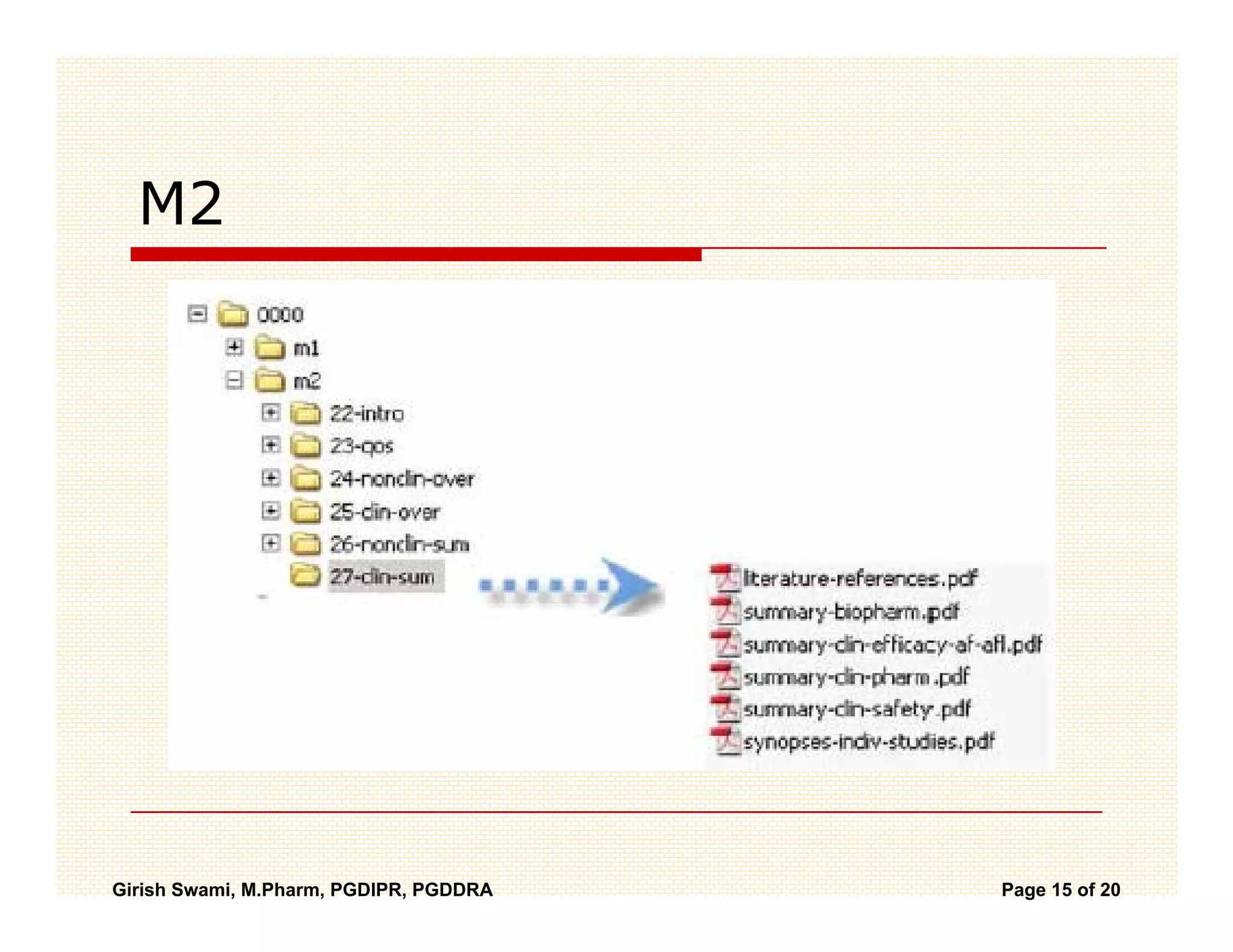
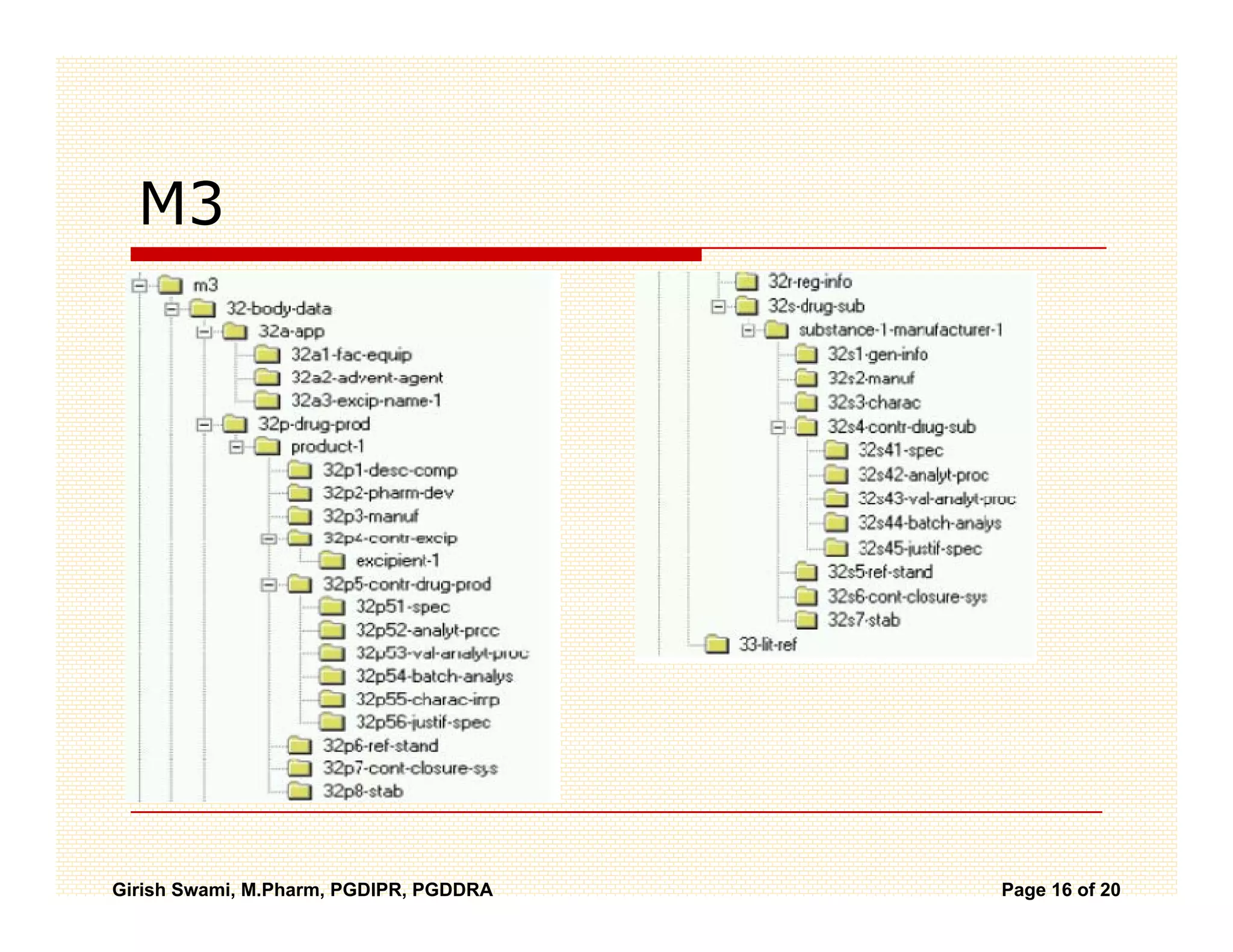
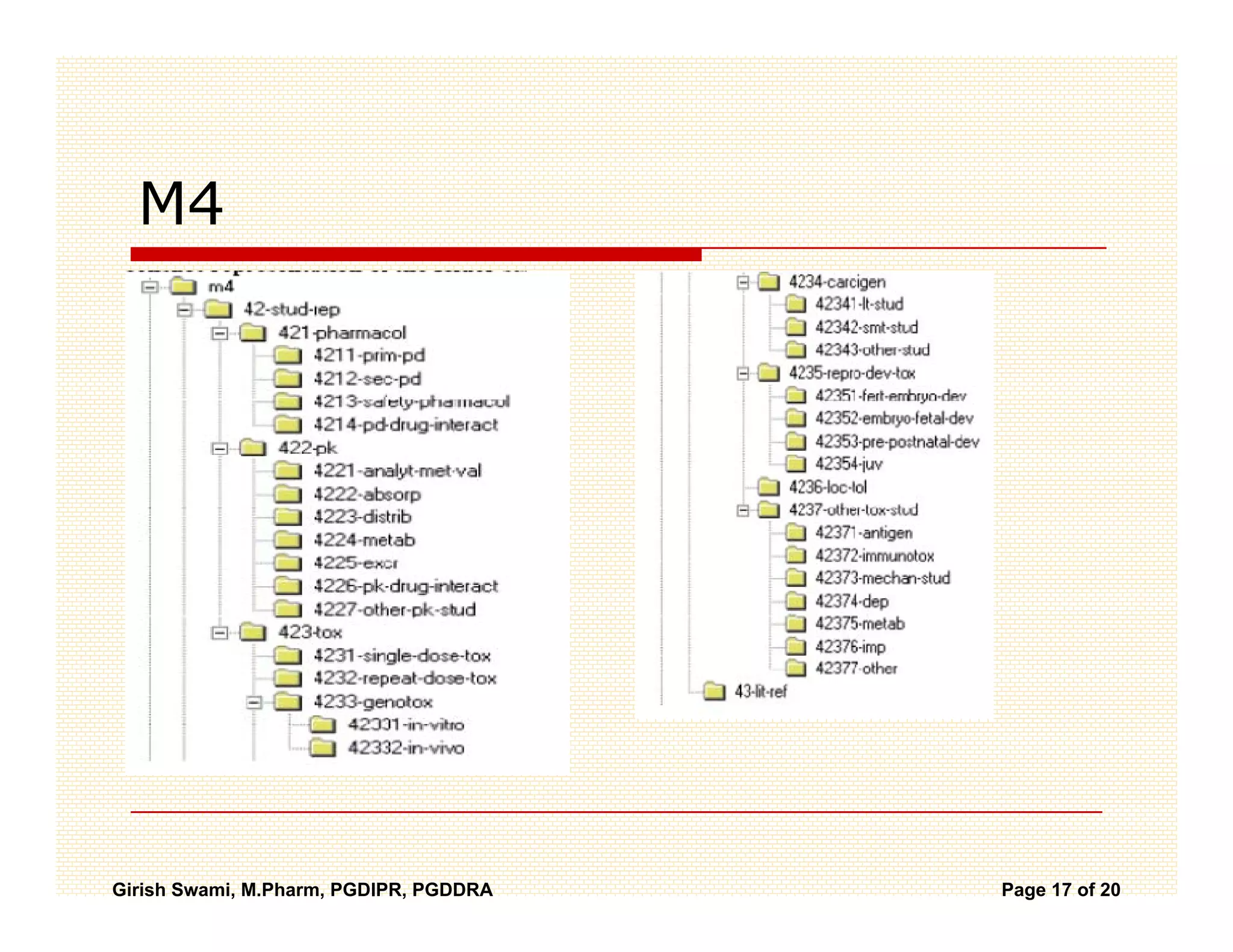
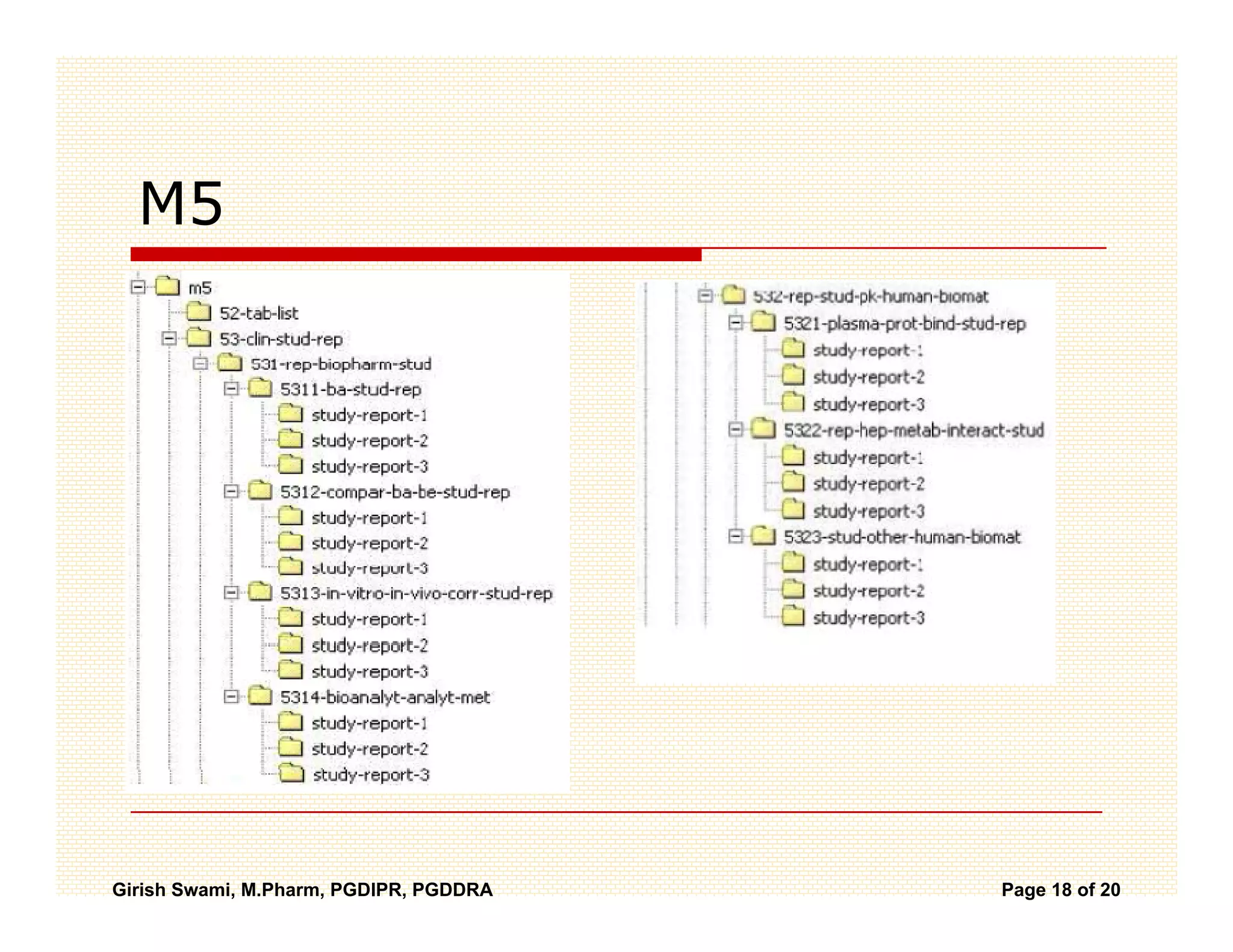
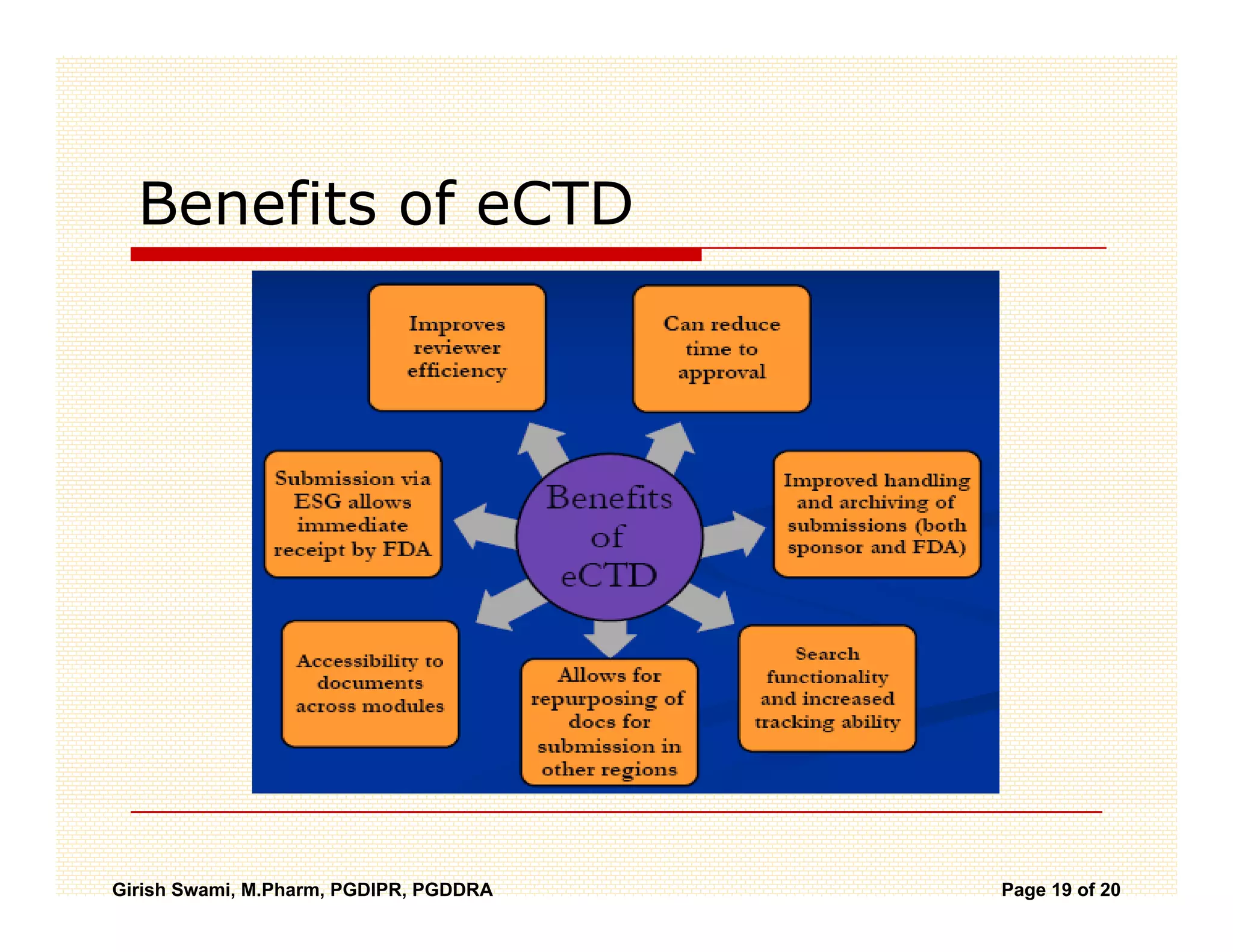
The Common Technical Document (CTD) is a standardized format for new drug applications agreed upon by international regulatory agencies. It has 5 modules covering administrative information, summaries, quality, non-clinical studies, and clinical studies. The electronic CTD (eCTD) is the electronic version that regulatory agencies now require. eCTD submissions have granular documents linked by XML and allow for increased transparency, ease of review, and benefits like reduced submission time and costs.