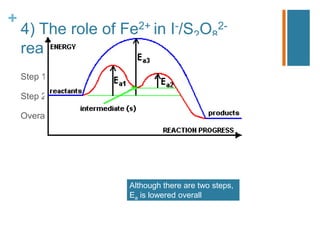
The document covers essential aspects of reaction kinetics, including rate of reaction, collision theory, and the effects of temperature and catalysts. It explains how to determine the order of reactions experimentally and details various methods for studying reaction rates. Additionally, it includes examples of catalysts in industrial processes and environmental applications, such as the Haber process and catalytic converters.





![+
Orders of reaction
Suppose of you have: A + B products
From the experiment: you found out that….
When [A] doubles, rate doubles.
Rate of reaction is proportional to [A]
Therefore, order with respect to A is 1
When [A] doubles, rate increases four times
Rate of reaction is proportional to [A]2
Therefore, order of reaction w.r.t. A is 2
When [A] doubles, rate of reaction does not change
Rate of reaction does not depend on [A]
Therefore, order w.r.t A is 0
WARNING:
YOU CANNOT DEDUCE THE ORDER OF
REACTION JUST BY LOOKING AT THE
EQUATION!!!!
ORDERS OF REACTIONS ARE ALWAYS FOUND
BY DOING EXPERIMENTS](https://image.slidesharecdn.com/chemicalkinetics-140512055311-phpapp01/85/Chemical-kinetics-6-320.jpg)
![+
Rate equation
Suppose A + 2B + C products
From experiments, we found out that:
[A] doubles, rate of reaction doubles
Therefore, order w.r.t A is 1
[B] doubles, rate of reaction increases
by 4
Therefore, order w.r.t B is 2
[C] doubles, rate of reaction does not change
Therefore, order w.r.t C is 0
Rate Equation:
Rate = k [A] [B]2
Where k is rate constant](https://image.slidesharecdn.com/chemicalkinetics-140512055311-phpapp01/85/Chemical-kinetics-7-320.jpg)

![+
Rate constant, k
Rate = k [A] [B]
Rate constant is constant (does not change value) only when
concentrations of reactants are changing.
RATE CONSTANT CHANGES WHEN:
1. TEMPERATURE CHANGES
2. ADDING CATALYST](https://image.slidesharecdn.com/chemicalkinetics-140512055311-phpapp01/85/Chemical-kinetics-9-320.jpg)
![+
DEDUCING ORDER BY INTIAL
RATES METHOD
Run Initial [A]/mol Initial [B]/mol Initial rate/mols-1
1 1.00 1.00 1.25 x 10-2
2 1.00 2.00 2.5 x 10-2
3 2.00 2.00 2.5 x 10-2
Can you find the rate equation? Can you find k?
Make [A] constant,
[B] x 2, rate x 2
Order w.r.t B is 1
Make [B] constant,
[A] x 2, rate same
Order w.r.t A is 0
Rate = k [B]
k=1.25 x 10-2 s-1](https://image.slidesharecdn.com/chemicalkinetics-140512055311-phpapp01/85/Chemical-kinetics-10-320.jpg)