More Related Content
More from fakhrobomba (20)
Phx53
- 1. 1
أدرار ﻟﻮﻻﯾﺔ اﻟﺘﺮﺑﯿﺔ ﻣﺪﯾﺮﯾﺔ
اﻟﺠﺪﯾﺪة اﻟﺜﺎﻧﻮﯾﺔ
–
أوﻟﻒ
: اﻟﺪراﺳﻲ اﻟﻤﻮﺳﻢ
2015 – 2014
*****ا
اﻟﻔﯿﺰﯾﺎﺋﯿﺔ اﻟﻌﻠﻮم ﻣﺎدة ﻓﻲ اﻷول اﻟﻔﺼﻞ ﻣﺘﺤﺎن
*****
: اﻟﺸﻌﺒﺔ
3
اﻟ اﻟﻤﺪة رﯾﺎﺿﻲ ﺗﻘﻨﻲ
: ﺰﻣﻨﯿﺔ
4
ﺳﺎﻋﺎت
----------------------------------------------------------------------------------------------------------------
: اﻷول اﻟﺘﻤﺮﯾﻦ
)
03
( ﻧﻘﺎط
إ
ﻗﺬف ن
أ
اﻵزوت ﻧﻮﯾﺔ
ﺗﺸﻜﻞ اﻟﻰ ﯾﺆدي ﻧﯿﺘﺮوﻧﺎت ﺑﻮاﺳﻄﺔ
أ
اﻟﻜﺮﺑﻮن ﻧﻮﯾﺔ
14
اﻟ ﺣﺴﺐ
ﺘﻔﺎﻋﻞ
: اﻟﺘﺎﻟﯿﺔ ﺑﺎﻟﻤﻌﺎدﻟﺔ اﻟﻤﻨﻤﺬج اﻟﻨﻮوي
+
→
+
اﻟﻜﺮﺑﻮن أﻧﻮﯾﺔ ان
14
وﺗﺘﻔﻜﻚ ﻣﺸﻌﺔ
ﺑﺈﺻﺪار
وﻧﻮاة
.
اﻟﻜﺮﺑﻮن ﻋﻤﺮ ﻧﺼﻒ زﻣﻦ ﯾﻘﺪر.
14
ﺑـ
5570 ans
.
1
–
أ
–
ﻋﺮف
:
-
اﻟﻤﺸﻌﺔ اﻟﻨﻮاة
.
-
.اﻟﻌﻤﺮ ﻧﺼﻒ زﻣﻦ
ب
–
اﻟﻜﺮﺑﻮن ﺗﻔﻜﻚ ﻣﻌﺎدﻟﺔ اﻛﺘﺐ
14
اﻻﺷﻌﺎﻋﻲ اﻟﻨﺸﺎط ﻧﻮع ﺣﺪد
. اﻟﻤﻮاﻓﻖ
ﺟـ
-
اﻟﻌﻨﺼﺮ اﺳﻢ اﻋﻂ
.
2
–
/ أ
واﻟﺜﺎﺑﺖ اﻟﻌﻤﺮ ﻧﺼﻒ زﻣﻦ ﺑﯿﻦ ﺗﺮﺑﻂ اﻟﺘﻲ اﻟﻌﻼﻗﺔ ﺑﺮھﻦ
λ
.
/ ب
اﻟﺜﺎﺑﺖ ﻗﯿﻤﺔ اﺣﺴﺐ
λ
.
3
–
ﺳﻔﯿﻨ ﺻﻨﻊ ﺳﻨﺔ ﻟﻤﻌﺮﻓﺔ
ﺳﻨﺔ ﺑﺎﺣﺜﻮن ﻋﻠﯿﮭﺎ ﻋﺜﺮ ﻗﺪﯾﻤﺔ ﺔ
1983
، اﻟﺒﺤﺮ ﻗﺎع ﻓﻲ م
اﺳﺘﻌﻤﻠﻮا
اﻟﺘ طﺮﯾﻘﺔ
ﺄ
اﻟﻜﺮﺑﻮن ﺑﻮاﺳﻄﺔ رﯾﺦ
14
ﻟﻌﯿﻨﺔ
اﻻﺷﻌﺎﻋﻲ اﻟﻨﺸﺎط ان . ﻣﻨﮭﺎ ﺧﺸﺒﯿﺔ
A
ھﻮ اﻟﻌﯿﻨﺔ ﻟﮭﺬه ﺑﺎﻟﻨﺴﺒﺔ اﻟﻤﻘﺎس
12
واﺣﺪ ﻟﻐﺮام وذاﻟﻚ اﻟﺪﻗﯿﻘﺔ ﻓﻲ ﺗﻔﻜﻚ
( 1g )
. اﻟﻜﺮﺑﻮن ﻣﻦ
وﺑﺎﻟﻤﻘﺎﺑﻞ
ا ﻣﻦ واﺣﺪ ﻟﻐﺮام ﺑﺎﻟﻨﺴﺒﺔ ﻓﺎﻧﮫ
ا ﺛﻨﺄي دورة ﻓﻲ اﻟﻤﺴﺎھﻢ ﻟﻜﺮﺑﻮن
و
ﻟﺪﯾﻨﺎ ، اﻟﺠﻮ ﻓﻲ اﻟﻜﺮﺑﻮن ﻛﺴﯿﺪ
A0
ﯾﺴﺎوي
13.6
. اﻟﺪﻗﯿﻘﺔ ﻓﻲ ﺗﻔﻜﻚ
. اﻟﺰﻣﻦ ﻣﻊ اﻟﺨﺸﺐ ﻟﻌﯿﻨﺔ اﻻﺷﻌﺎﻋﻲ اﻟﻨﺸﺎط ﺗﻨﺎﻗﺺ ﻋﻠﻞ / أ
. ﺻﻨﻌﮭﺎ ﺳﻨﺔ اﺳﺘﻨﺘﺞ ﺛﻢ اﻛﺘﺸﺎﻓﮭﮫ وزﻣﻦ اﻟﺴﻔﯿﻨﺔ ﺻﻨﻊ زﻣﻦ ﺑﯿﻦ اﻟﻔﺎﺻﻠﺔ اﻟﺰﻣﻨﯿﺔ اﻟﻔﺘﺮة أﺣﺴﺐ / ب
اﻟﺜﺎﻧﻲ اﻟﺘﻤﺮﯾﻦ
:
)
04
ﻧﻘ
( ﺎط
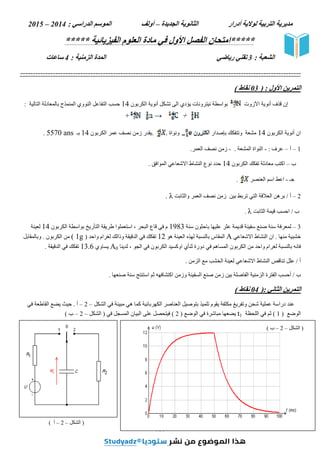
اﻟﺸﻜﻞ ﻓﻲ ﻣﺒﯿﻨﺔ ھﻲ ﻛﻤﺎ اﻟﻜﮭﺮﺑﺎﺋﯿﺔ اﻟﻌﻨﺎﺻﺮ ﺑﺘﻮﺻﯿﻞ ﺗﻠﻤﯿﺬ ﯾﻘﻮم ﻣﻜﺜﻔﺔ وﺗﻔﺮﯾﻎ ﺷﺤﻦ ﻋﻤﻠﯿﺔ دراﺳﺔ ﻋﻨﺪ
–
2
–
. أ
ﻓﻲ اﻟﻘﺎطﻌﺔ ﯾﻀﻊ ﺣﯿﺚ
اﻟﻮﺿﻊ
( 1 )
ﺛﻢ
اﻟﻠﺤﻈﺔ ﻓﻲ
t1
ﯾﻀﻌﮭﺎ
ﻣﺒﺎﺷﺮة
اﻟﻮﺿﻊ ﻓﻲ
( 2 )
ﻓﻲ اﻟﻤﺴﺠﻞ اﻟﺒﯿﺎن ﻋﻠﻰ ﻓﯿﺘﺤﺼﻞ
اﻟﺸﻜﻞ )
–
2
–
( ب
اﻟﺸﻜﻞ )
–
2
–
( ب
اﻟﺸﻜﻞ )
–
2
–
( أ
- 2. 2
I
-
در
:اﻟﺸﺤﻦ ﻋﻤﻠﯿﺔ اﺳﺔ
1
-
.اﻟﻤﻜﺜﻔﺔ طﺮﻓﻲ ﺑﯿﻦ اﻟﺘﻮﺗﺮ ﻟﮭﺎ ﯾﺨﻀﻊ اﻟﺘﻲ اﻟﺘﻔﺎﺿﻠﯿﺔ اﻟﻤﻌﺎدﻟﺔ اﻛﺘﺐ
2
-
. اﻟﻤﻌﺎدﻟﺔ ھﺬه ﺣﻞ أﻛﺘﺐ
3
-
: ﺑﯿﺎﻧﯿﺎ ﻋﯿﻦ
اﻟﻠﺤﻈﺔ (أ
t1
ﻗﯿﻤﺔ ( ب .
E
اﻟﺰﻣﻦ ﺛﺎﺑﺖ ( ﺟـ .
τ1
.
اﻟﺸﺤﻦ اﺗﻤﺎم ﻣﺪة ( د
T1
.
II
-
:اﻟﺘﻔﺮﯾﻎ ﻋﻤﻠﯿﺔ دراﺳﺔ
1
-
اﻟﺘﯿﺎر ﺟﮭﺔ وﺣﺪد اﻟﺘﻔﺮﯾﻎ دارة ﻣﺜﻞ
.
2
-
ﻋﯿﻦ
إﺗ ﻣﺪة ( أ : ﺑﯿﺎﻧﯿﺎ
اﻟﺘﻔﺮﯾﻎ ﻤﺎم
T2
اﻟﺰﻣﻦ ﺛﺎﺑﺖ ( ب .
τ2
.
3
-
ﻗﯿﻤﺔ ھﻞ
R1
ﺗﺴﺎوي أو ﻛﺒﺮ أو أﺻﻐﺮ
R2
. ﻋﻠﻞ ؟
III
-
ﻗﯿﻤﺔ
اﻟﻤﻘﺎوﻣﺔ
R1
ھﻲ
40 Ω
: ﺣﺴﺎب اﻟﻤﻄﻠﻮب .
1
–
اﻟﻤﻜﺜﻔﺔ ﺳﻌﺔ
C
.
2
–
اﻟﻤﻘﺎوﻣﺔ
R2
.
: اﻟﺜﺎﻟﺚ اﻟﺘﻤﺮﯾﻦ
)
3
0
( ﻧﻘﺎط
اﻟﮭﯿﺪ ﻛﻠﻮر ﺣﻤﺾ ﯾﺆﺛﺮ
روﺟﯿﻦ
( H+
+ Cl -
)( aq )
اﻷﻟﻤﻨﯿﻮم ﻣﻌﺪن ﻋﻠﻰ
( Al(s) )
: اﻟﺘﺎﻟﯿﺔ اﻟﻤﻌﺎدﻟﺔ ﺣﺴﺐ
6H+
(aq ) + 6 Cl-
(aq) + 2Al(s) = 3H2(g) + 2Al3+
+ 6Cl-
(aq)
اﻟﻠﺤﻈﺔ ﻓﻲ
t = 0
ﻛﺘﻠﺔ ﻧﺪﺧﻞ
m = 1,20 g
ﺗﺤﺘﻮ ﺣﻮﺟﻠﺔ ﻓﻲ اﻷﻟﻤﻨﯿﻮم ﺑﺮادة ﻣﻦ
ﺣﺠﻢ ﻋﻠﻰ ي
VA = 60,0 mL
ﻛﻠﻮر ﺣﻤﺾ ﻣﻦ
اﻟﻤﻮﻟﻲ ﺗﺮﻛﯿﺰه اﻟﺬي اﻟﮭﯿﺪروﺟﯿﻦ
CA = 0,15 mol / L
ﯾﻌﻄﻰ .
M( Al ) = 27 g/mol
.
1
–
؟ اﻟﺘﻔﺎﻋﻞ ﻓﻲ اﻟﺪاﺧﻠﺘﯿﻦ اﻟﺜﻨﺎﺋﯿﺘﯿﻦ اﺳﺘﻨﺘﺞ ؟ اﻟﺘﺤﻮل ھﺬا ﻣﺎﻧﻮع
2
–
اﻟ ﻟﻠﺘﺤﻮل اﻟﺘﻘﺪم ﺟﺪول أﻧﺸﺊ
اﻟﻤﺤﺪ اﻟﻤﺘﻔﺎﻋﻞ اﺳﺘﻨﺘﺞ ﺛﻢ ﻤﺪروس
و
ﻗﯿﻤﺔ
Xmax
؟
3
–
ﻧ
اﻟﺒﯿﺎن ﺑﺮﺳﻢ ﺳﻤﺤﺖ اﻟﻨﺘﺎﺋﺞ اﻟﺰﻣﻦ ﺑﺪﻻﻟﺔ اﻷﻟﻤﻨﯿﻮم ﻟﺸﻮارد اﻟﻤﻮﻟﻲ اﻟﺘﺮﻛﯿﺰ ﻟﺘﻄﻮر اﻟﺰﻣﻨﯿﺔ ﺑﺎﻟﻤﺘﺎﺑﻌﺔ ﻘﻮم
اﻟﺸﻜﻞ ﻓﻲ اﻟﻤﻮﺿﺢ
–
4
-
:
/ أ
ﻟﻈﮭﻮر اﻟﺤﺠﻤﯿﺔ اﻟﺴﺮﻋﺔ أﺣﺴﺐ
Al 3+
اﻟﻠﺤﻈﺔ ﻋﻨﺪ
t = 200 s
و
t = 0 s
.
/ ب
.
اﻟﺘﻔﺎﻋﻞ ﺳﺮﻋﺔ اﺳﺘﻨﺘﺞ
Vr
ﻋﻨﺪ
t = 200s
.
ﺟـ
/
؟ اﻟﺘﻐﯿﺮ ھﺪا ﻋﻦ اﻟﻤﺴﺆول اﻟﺤﺮﻛﻲ اﻟﻌﺎﻣﻞ ﻣﺎھﻮ . اﻟﺘﻔﺎﻋﻞ ﺳﺮﻋﺔ ﺗﺘﻐﯿﺮ ﻛﯿﻒ
ﺍﻟﺷﻛﻝ
–
4
-
- 3. 3
: اﻟﺮاﺑﻊ اﻟﺘﻤﺮﯾﻦ
)
03
( ﻧﻘﺎط
اﻟﯿﻮراﻧﯿﻮم اﻧﺸﻄﺎر ﻋﻦ اﻟﻨﺎﺗﺠﺔ اﻟﻨﻮوﯾﺔ اﻟﻄﺎﻗﺔ ﻛﻮﻗﻮد ﺗﺴﺘﻌﻤﻞ اﻟﻐﻮاﺻﺎت ﺑﻌﺾ
235
ﻣﻤﻜﻨﺔ اﻧﺸﻄﺎر ﺗﻔﺎﻋﻼت ﻋﺪة ﯾﻮﺟﺪ .
ﻟﻠﯿﻮراﻧﯿﻮم
235
،
ﻛﻞ ان ﻧﻔﺘﺮض وﻟﻜﻦ
اﻹﻧﺸﻄﺎرات
: اﻟﺘﺎﻟﯿﺔ اﻟﻤﻌﺎدﻟﺔ ﺣﺴﺐ ﺗﻜﻮن اﻟﻐﻮاﺻﺔ ھﺬه ﻓﻲ ﺗﺤﺪث اﻟﺘﻲ
+ → + + 2
1
–
اﻟﻌﺪدﯾﻦ ﺟﺪ اﻹﻧﺤﻔﺎظ ﻗﺎﻧﻮﻧﻲ ﺑﺘﻄﺒﯿﻖ
x
و
y
.
2
-
ﺑـ أﺣﺴﺐ
MeV
اﻟ ﺑﺎﻟﺠﻮل ﺛﻢ
ﻋﻦ اﻟﻤﺤﺮرة ﻄﺎﻗﺔ
ﻧﻮا اﻧﺸﻄﺎر
ة
اﻟﯿﻮراﻧﯿﻮم ﻣﻦ واﺣﺪة
235
.
3
–
أﺣﺴﺐ
ﺑﺎﻟﺤﻮل
اﻧﺸﻄﺎر ﻋﻦ اﻟﻤﺤﺮرة اﻟﻄﺎﻗﺔ
235 g
اﻟﯿﻮارﻧﯿﻮم ﻣﻦ
235
.
4
–
ﻣﻦ واﺣﺪ ﯾﻮم ﻓﻲ اﻟﻤﺴﺘﮭﻠﻜﺔ اﻟﯿﻮراﻧﯿﻮم ﻛﺘﻠﺔ أﺣﺴﺐ
.اﺷﺘﻐﺎل
ﻗﺪرھﺎ اﺳﺘﻄﺎﻋﺔ ﻟﻠﻐﻮاﺻﺔ ﯾﻘﺪم اﻟﻐﻮاﺻﺔ ﻣﻔﺎﻋﻞ أن ﻋﻠﻤﺖ اذا
10MW
.
ھﻮ اﻟﻤﻔﺎﻋﻞ وﻣﺮدود
r = 33 %
.
: اﻟﻤﻌﻄﯿﺎت
m( ) = 235,0439 u , m( ) = 139,9252 u , m( ) = 93,9154 u
m( ) = 1,0087 u
،
NA = 6.10 23
mol-1
،
1u = 931,5 MeV/C2
: اﻟﺨﺎﻣﺲ اﻟﺘﻤﺮﯾﻦ
)
03
( ﻧﻘﺎط
- 4. 4
: اﻟﺘﺠﺮﯾﺒﻲ اﻟﺘﻤﺮﯾﻦ
)
4
0
( ﻧﻘﺎط
اﻟﺘﻼﻣﯿﺬ ﻣﻦ ﻣﺠﻤﻮﻋﺔ ﺗﺪرس
ﺗﻔﺎﻋﻞ
2
–
ﺑﺮوﻣﻮ
-
2
–
ﺑﺮوﺑﺎن ﻣﯿﺜﯿﻞ
( CH3)3CBr
: ﻣﻌﺎدﻟﺘﮫ ﺗﺎم ﺗﻔﺎﻋﻞ وﻓﻖ اﻟﻤﺎء ﻣﻊ
RBr + H2O → ROH + H+
(aq) + Br-
(aq)
وا ﻧﺮﻣﺰ ﺣﯿﺚ
ل ﻧﺮﻣﺰ ﻟﺬي
2
–
ﺑﺮوﻣﻮ
-
2
–
ﺑﺮوﺑﺎن ﻣﯿﺜﯿﻞ
( CH3)3CB
ﺑـ
RBr
اﻟﺤﺮارة درﺟﺔ ﻋﻨﺪ اﻟﺘﺠﺮﺑﺔ ﺗﺠﺮى
Ɵ = 25°C
ﯾ
ﺤﻀﺮ
اﻟﺘﻼﻣﯿﺬ أﺣﺪ
ﺣﺠﻤﺎ ﻣﻦ ﯾﺘﻜﻮن ﻣﺰﯾﺠﺎ
V( eau ) = 100 mL
اﻟﻤﻘﻄﺮ اﻟﻤﺎء ﻣﻦ
وﺣﺠﻤﺎ
V( RBr ) = 1 mL
، اﻷﺳﺘﻮن ﻣﻦ وﻗﻠﯿﻼ
اﻟﻤﺰج ﻟﺤﻈﺔ ﻓﻲ
t = 0
ﯾﺘﻢ
ﻗﯿ
ﺎ
اﻟﻤﺰﯾﺞ ﻧﺎﻗﻠﯿﺔ س
اﻟﻨﺎﻗﻠﯿﺔ ﻣﻘﯿﺎس ﺑﻮاﺳﻄﺔ
ﺧﻠﯿﺘﮫ ﺛﺎﺑﺖ
K
= 0,01 m
ﻛﻞ ﺑﻌﺪ ﺛﻢ ،
10
ﻟﻠﻨﺎﻗﻠﯿﺔ اﻟﺠﺪﯾﺪة اﻟﻘﯿﻤﺔ اﻟﺘﻼﻣﯿﺬ ﯾﺴﺠﻞ دﻗﺎﺋﻖ
ﻧﺘﺎﺋﺞ
اﻟﻘﯿﺎﺳﺎت
: اﻟﺘﺎﻟﻲ اﻟﺠﺪول ﻓﻲ ﻣﺪوﻧﺔ
100
90
80
70
60
50
40
30
20
10
0
t ( mn )
640
640
640
630
610
590
560
500
400
240
0
G( mS )
1
–
أ
/
ﯾﻤﻜ ﻟﻤﺎذا
ھ ﺗﻄﻮر ﺗﺘﺒﻊ ﻦ
؟ اﻟﻨﺎﻗﻠﯿﺔ ﻗﯿﺎس ﺑﻮاﺳﻄﺔ اﻟﺘﺤﻮل ﺬا
. اﻟﺘﺤﻮل ھﺬا ﺗﻄﻮر ﺗﺘﺒﻊ ﻣﻦ ﺗﻤﻜﻦ أﺧﺮى طﺮﯾﻘﺔ أﻋﻂ / ب
2
–
أﺣﺴﺐ /أ
n0
ﻟـ اﻹﺑﺘﺪاﺋﯿﺔ اﻟﻜﻤﯿﺔ
RBr
.
: ﻣﻌﻄﯿﺎت
اﻟﻜﺜﺎﻓﺔ
d ( RBr ) = 0,87
،
M( RBr ) = 136,9 g/mol
،
ρ( eau ) = 1 g /mL
/ ب
أﻧﺠﺰ
. اﻟﺘﻔﺎﻋﻞ ﺗﻘﺪم ﺟﺪول
3
–
أ
-
اﻟﺘﻔﺎﻋﻞ ﺗﻘﺪم ﺑﺪﻻﻟﺔ اﻟﺘﺤﻮل أﺛﻨﺎء اﻟﻤﺰﯾﺞ ﻧﺎﻗﻠﯿﺔ ﻋﻦ ﻋﺒﺮ
x
اﻟﻤﺰﯾﺞ ﺣﺠﻢ
V
،
K
،
λ( H+
)
،
λ( Br-
)
ب
–
ﺑﺪﻻﻟﺔ ﻟﻠﺘﻔﺎﻋﻞ اﻟﺤﺠﻤﯿﺔ اﻟﺴﺮﻋﺔ ﻋﻦ ﻋﺒﺮ
G( t )
،
K
،
λ( H+
)
،
λ( Br-
)
.
ج
–
اﻟﻨﮭﺎﺋﯿﺔ اﻟﺤﺎﻟﺔ ﻓﻲ اﻟﻤﺰﯾﺞ ﻧﺎﻗﻠﯿﺔ ﻋﻦ ﻋﺒﺮ
Gf
ﺑﺪﻻﻟﺔ
n0
،
V
،
K
،
λ( H+
)
،
λ( Br-
)
.
د
-
ﺑﯿﻦ
ان
x(t ) = n0 .
.
4
–
ﻣﻨﺤﻨﻰ أرﺳﻢ / أ
G = f(t )
: اﻟﺴﻠﻢ ﺑﺎﺳﺘﻌﻤﺎل
1cm → 10 mn
t :
و
1cm → 100 mS
G :
/ ب
. اﻟﺘﻔﺎﻋﻞ ﻧﺼﻒ زﻣﻦ ﻗﯿﻤﺔ ﺣﺪد
5
-
اﻟﺤﺮارة درﺟﺔ ﻋﻨﺪ اﻟﺘﺤﻮل ﻧﻔﺲ ﺑﺪراﺳﺔ اﻟﺘﻼﻣﯿﺬ ﻣﻦ اﺧﺮى ﻣﺠﻤﻮﻋﺔ ﻗﺎﻣﺖ
Ɵ = 45° C
ﻓ .
: اﻟﺘﺎﻟﯿﺔ اﻟﻨﺘﺎﺋﺞ ﺘﺤﺼﻠﻮﻋﻞ
: اﻟﺘﻔﺎﻋﻞ ﻧﺼﻒ زﻣﻦ
t1/2 = 7,0 mn
: ﻟﻠﻨﺎﻗﻠﯿﺔ اﻟﻨﮭﺎﺋﯿﺔ اﻟﻘﯿﻤﺔ و
Gf = 680 mS
/ أ
ﺗ ﻛﯿﻒ ﻣﯿﻜﺮوﺳﻜﻮﺑﯿﺎ ﻓﺴﺮ
ﺘ
. اﻟﺤﺮارة درﺟﺔ ﺑﺘﻐﯿﺮ اﻟﺘﻔﺎﻋﻞ ﺳﺮﻋﺔ ﻐﯿﺮ
ﻟﻢ ﻟﻤﺎذا ﻓﺴﺮ / ﺑـ
اﻟﻤﺠﻤﻮﻋﺘﯿﻦ ﺗﺘﺤﺼﻞ
ﻧﻔﺲ ﻋﻠﻰ
اﻟﻨﮭﺎﺋﯿﺔ اﻟﺤﺎﻟﺔ
.
ﺑ *******************
********************** ﻟﻠﺠﻤﻴﻊ ﺎﻟﺘﻮﻓﻴﻖ