Embed presentation
Downloaded 23 times


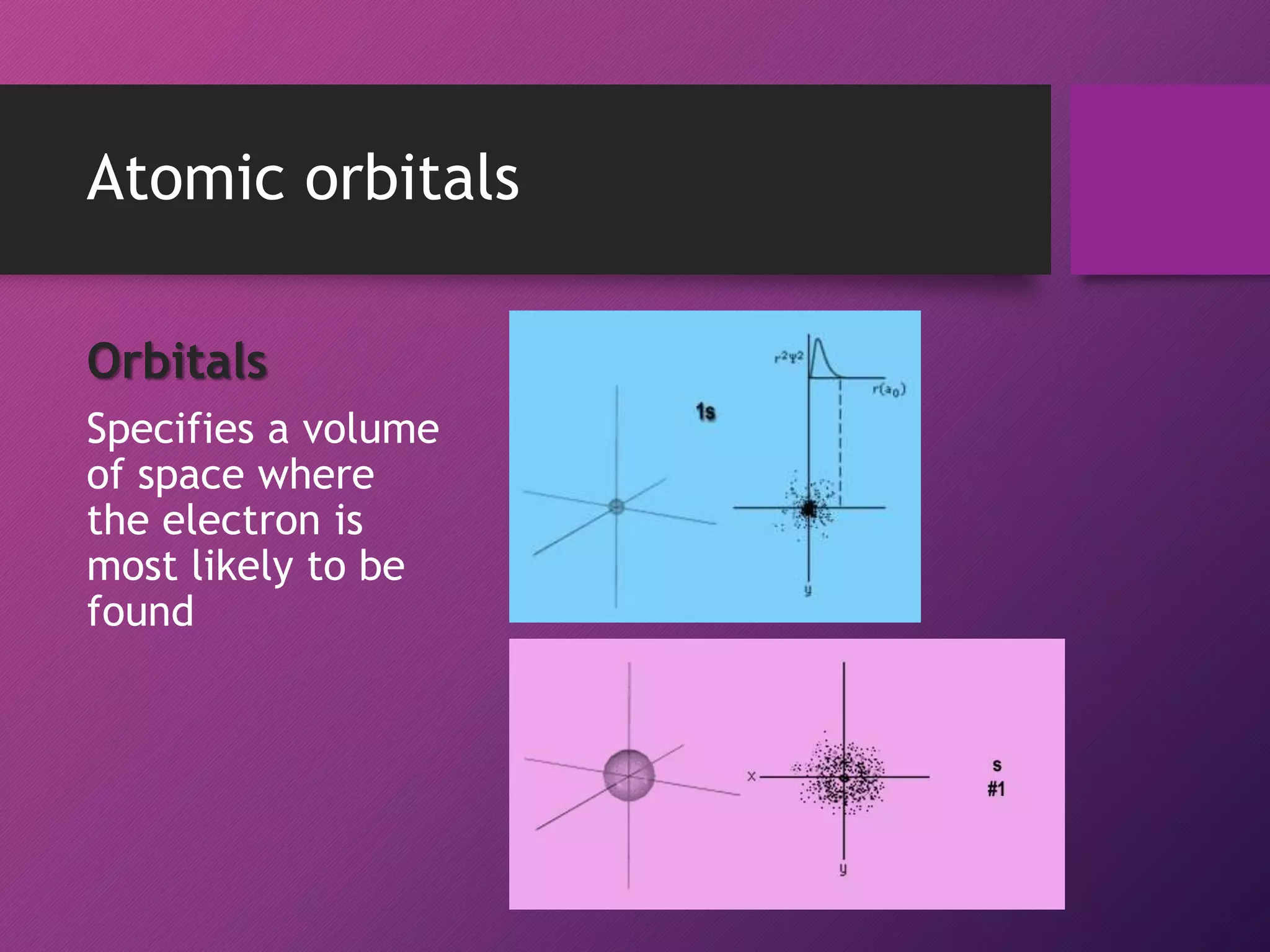
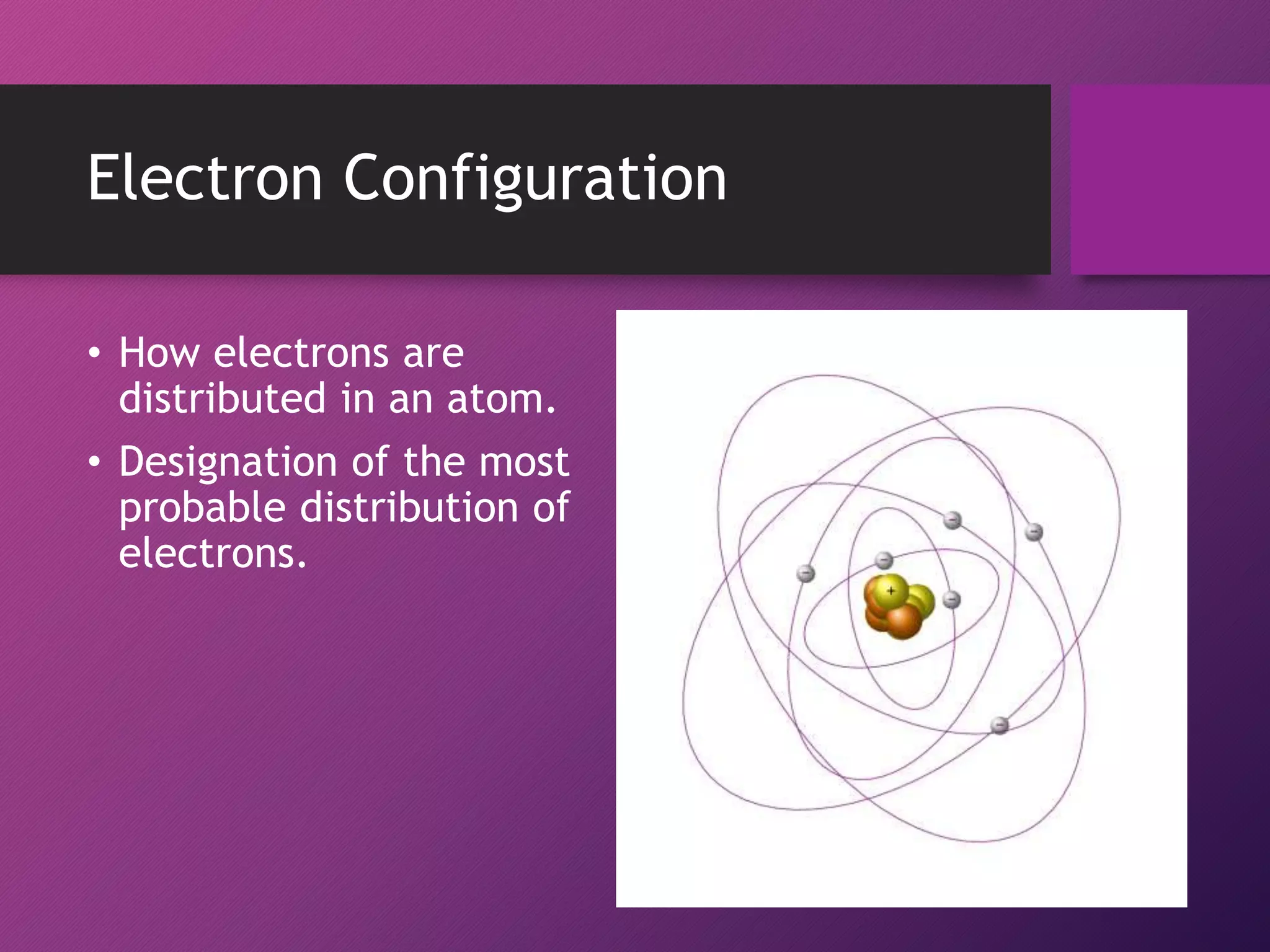
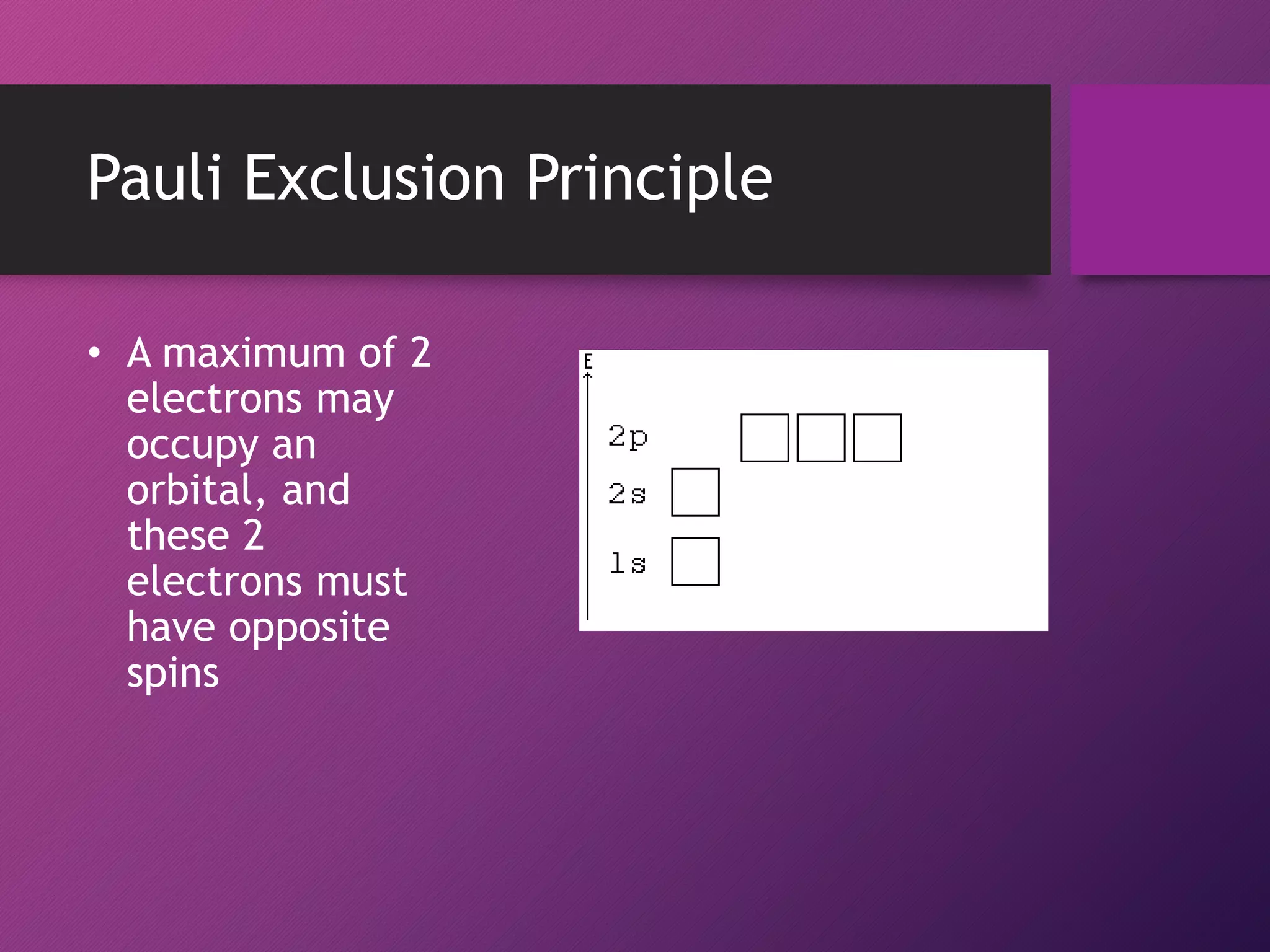
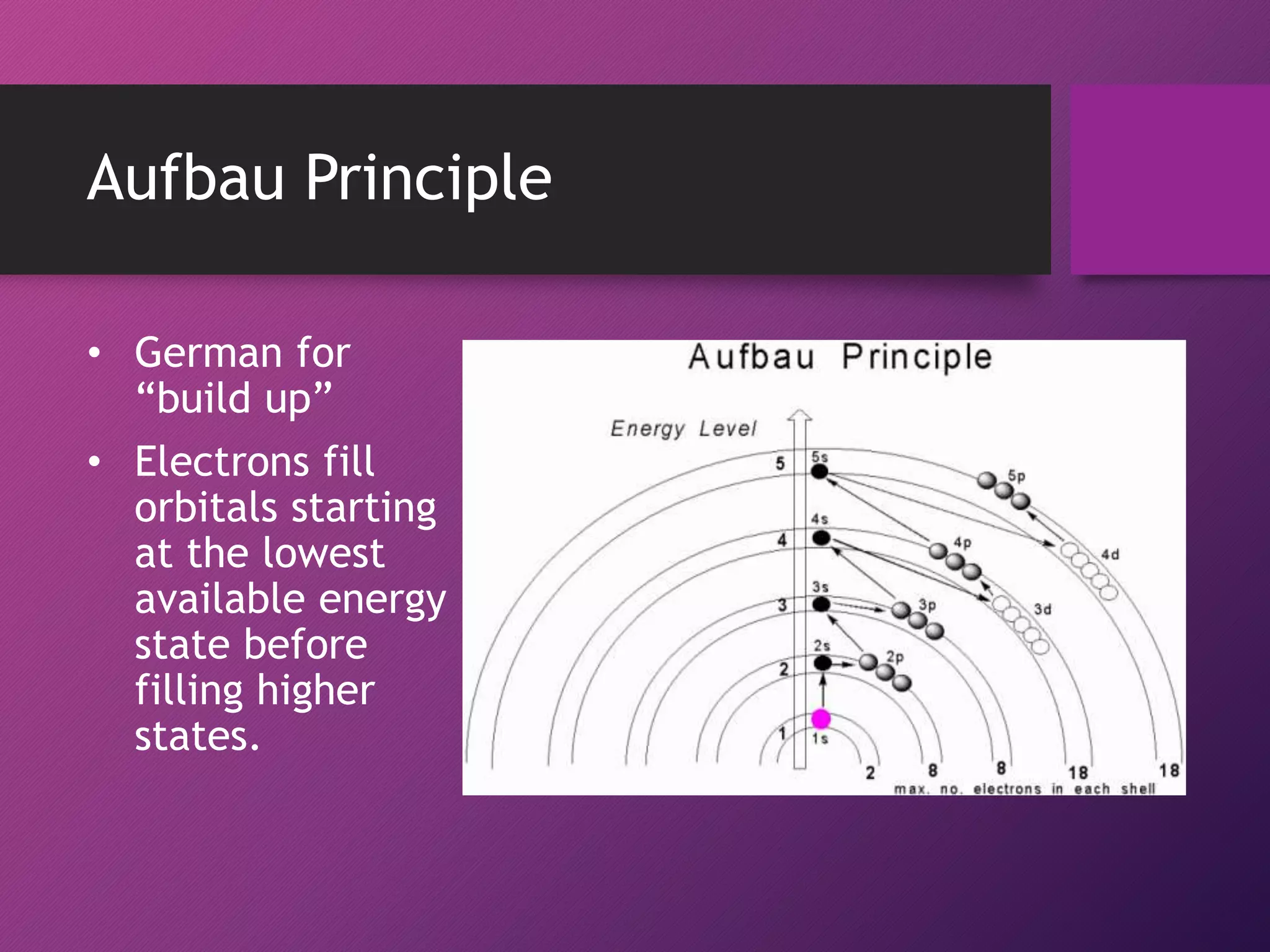
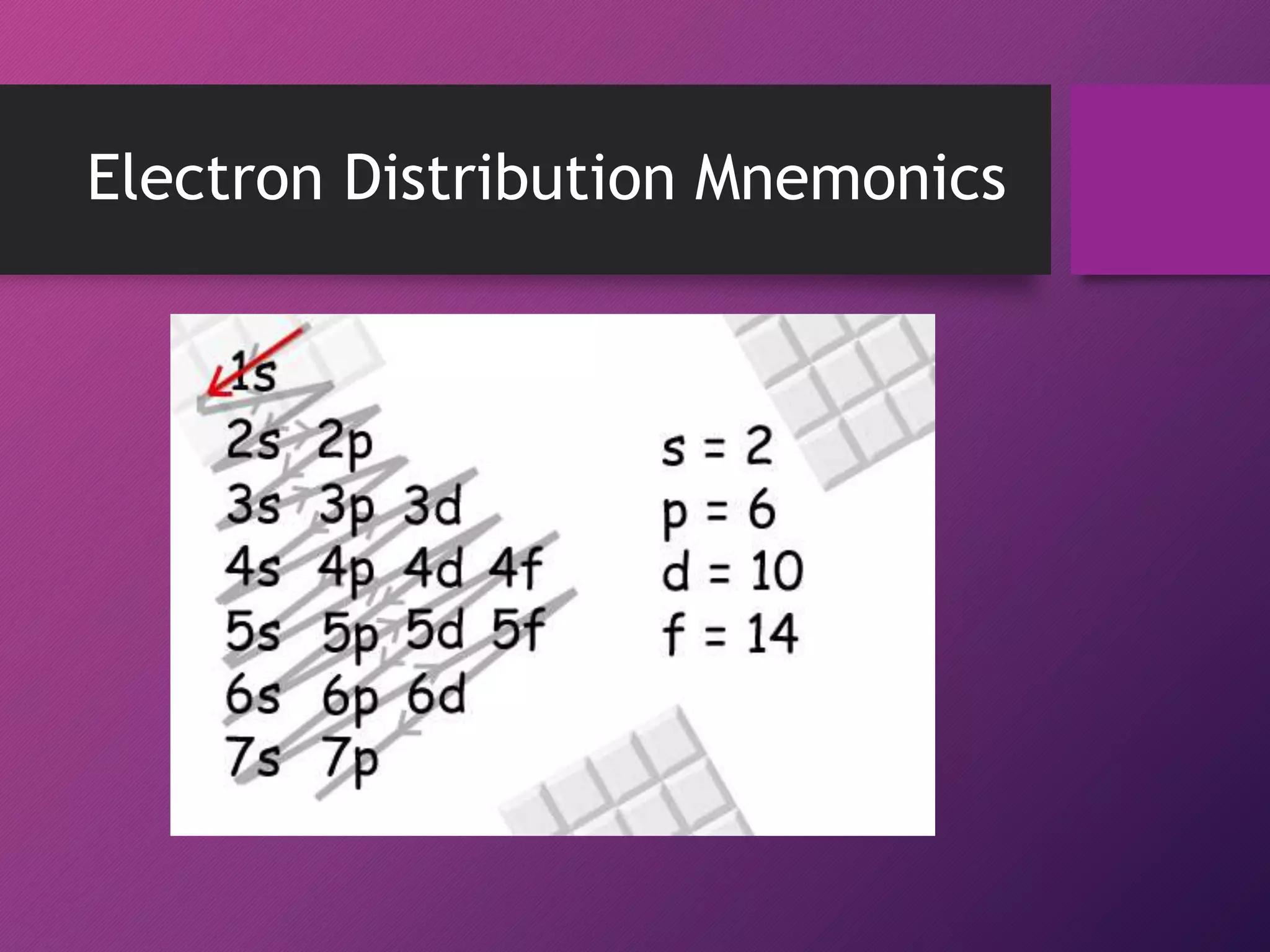




This document discusses electron configuration and the principles that govern how electrons are arranged in atoms. It explains that electrons exhibit wave-like properties and occupy regions of space called atomic orbitals. The distribution of electrons in atoms, known as electron configuration, follows three main principles: the Pauli Exclusion Principle limits orbitals to two electrons of opposite spin; the Aufbau Principle states that orbitals are filled from lowest to highest energy; and Hund's Rule specifies that orbitals are singly occupied with parallel electron spins before double occupation occurs.










