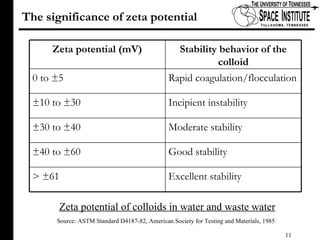
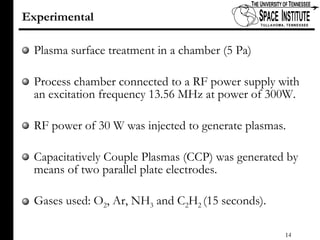
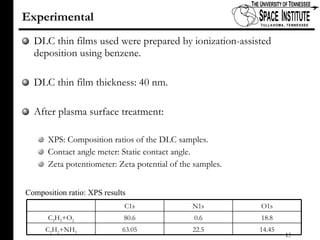
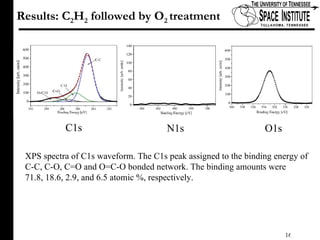
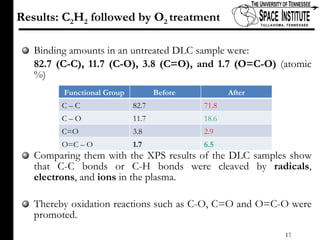
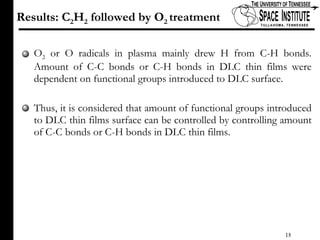
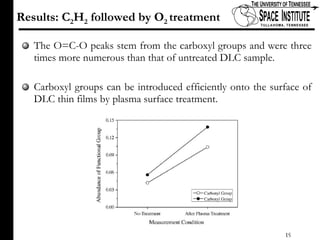
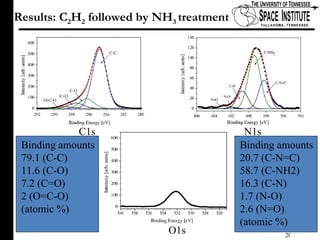
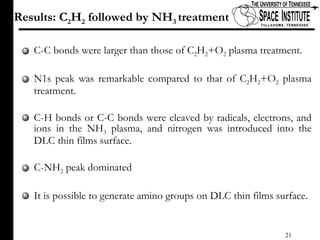
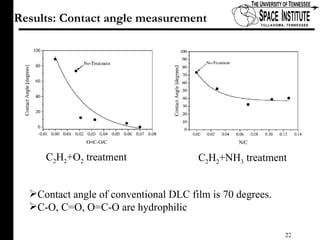
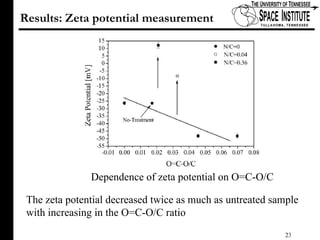
The document discusses diamond-like carbon (DLC) thin films, emphasizing their potential medical applications due to properties like biocompatibility and antithrombogenicity. It highlights the significance of controlling zeta potential through plasma surface treatment to improve compatibility with biological environments, particularly for blood-contacting medical devices. Experimental results demonstrate the effectiveness of introducing functional groups to manipulate zeta potential, showcasing a novel approach to enhance the performance of DLC materials in medical settings.
![Diamond-like Carbon Thin Film with Controlled Zeta Potential for Medical Application [Nitta et. al., Diamond & Related Materials 17 (2008) 1972-1976] MSE 576 Thin Films & Analysis Presentation Dec 4 th 2008 Deepak Rajput Graduate Research Assistant Center for Laser Applications University of Tennessee Space Institute Tullahoma, Tennessee 37388-9700 Email: [email_address] Web: http://drajput.com](https://image.slidesharecdn.com/zetapotential-091218161901-phpapp02/75/Zeta-Potential-1-2048.jpg)