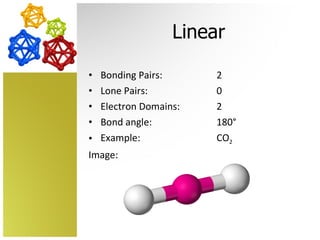
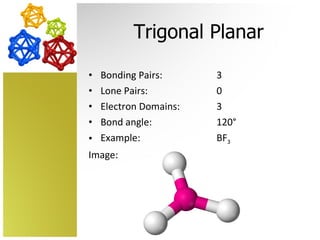
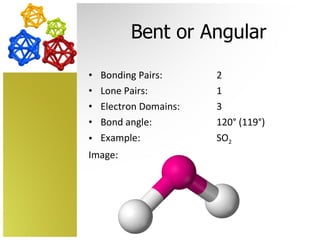
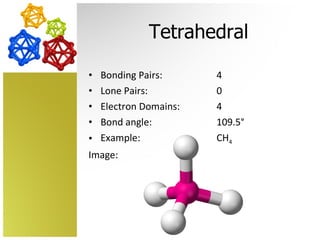
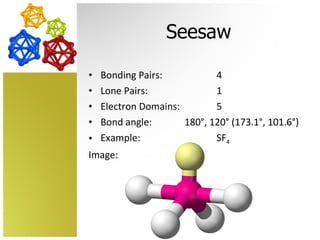
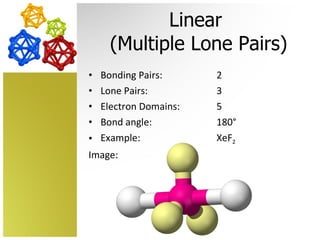
The document discusses molecular geometry and how the shapes of molecules can be predicted using the valence shell electron pair repulsion (VSEPR) model. It defines key terms like bonding pairs, lone pairs, and electron domains. It then provides examples of different molecular shapes like linear, trigonal planar, tetrahedral, and octahedral along with example molecules and diagrams.