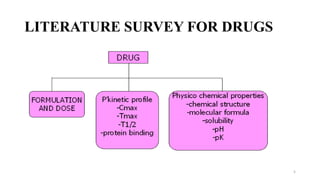
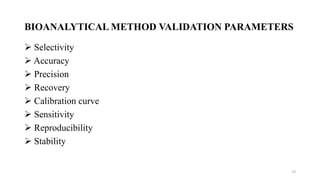
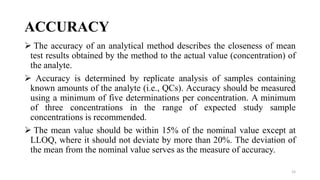
The document discusses guidelines for bioanalytical method validation from the USFDA. It describes key parameters that must be validated for a bioanalytical method, including selectivity, accuracy, precision, recovery, calibration curves, sensitivity, reproducibility and stability. Accuracy and precision are determined by analyzing quality control samples in replicates across multiple runs. Recovery experiments compare extracted samples to unextracted standards. A calibration curve consisting of multiple concentrations over the expected range must be precise and reproducible.