Embed presentation
Download as PDF, PPTX



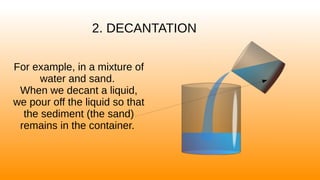







Magnetic separation uses magnets to separate mixtures where one substance is attracted to magnets and the other is not, such as separating iron filings from sand. Decantation involves pouring off the liquid layer from a mixture like water and sand so that the denser sediment remains behind. Filtration uses a filter to catch solid particles in a mixture while allowing the liquid to pass through, such as filtering coffee grounds from water.









