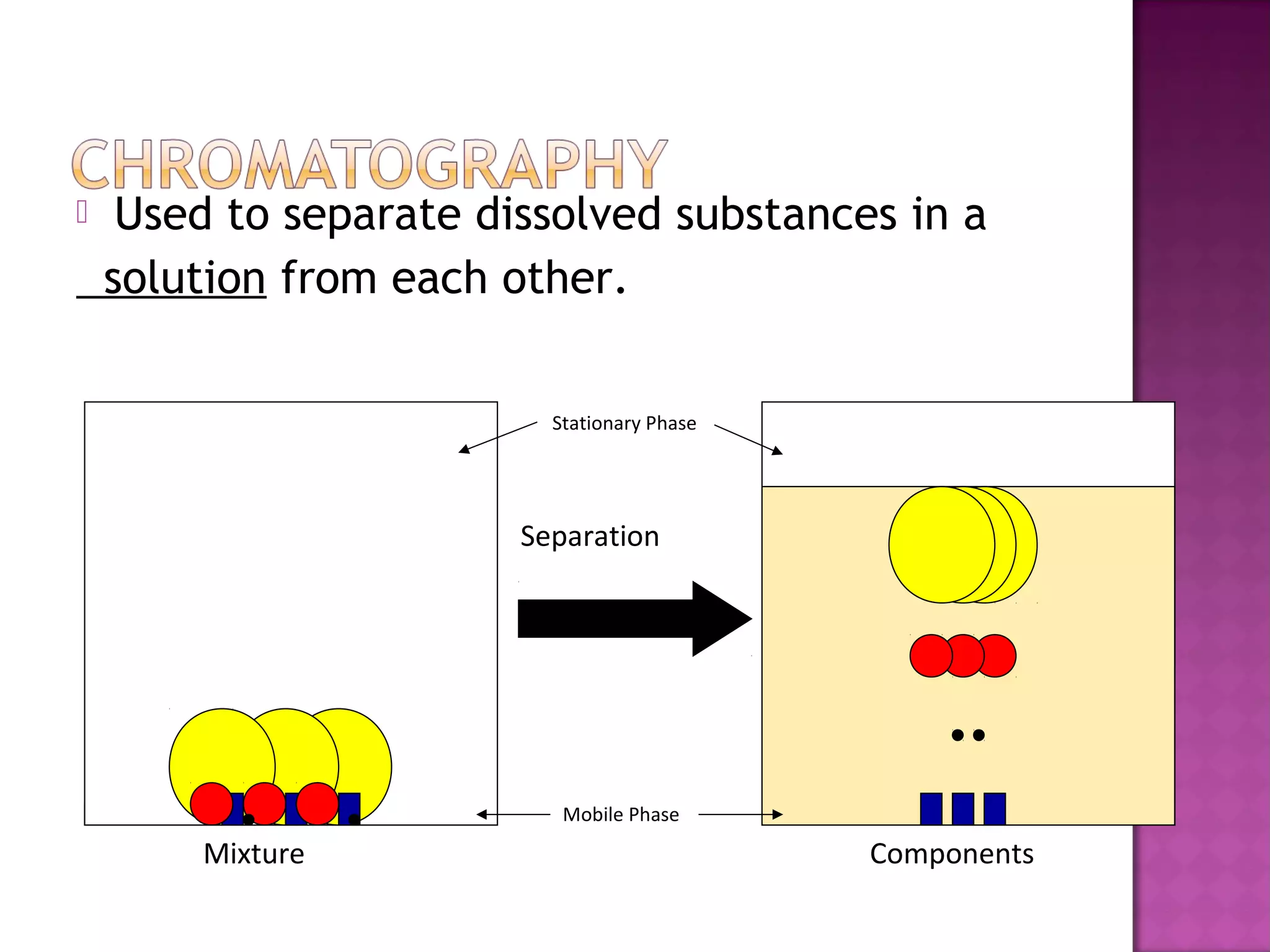
This document discusses various physical separation techniques for mixtures. It defines a mixture as two or more substances mixed together without chemical bonding that retain their individual properties and can be separated physically. Some common separation methods described are magnetism, hand separation, filtration, sifting, extraction and evaporation, and chromatography. Specific examples are provided to illustrate each technique.