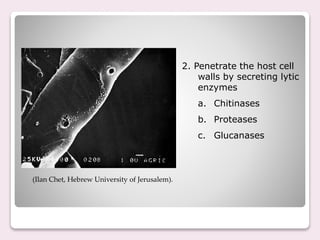
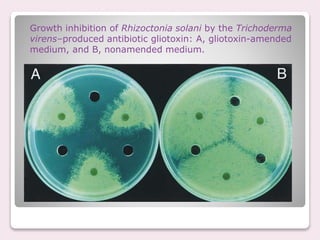
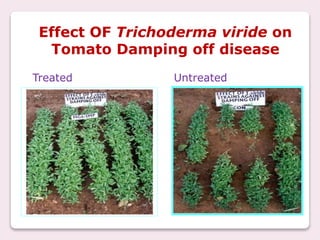
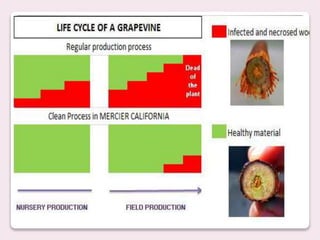
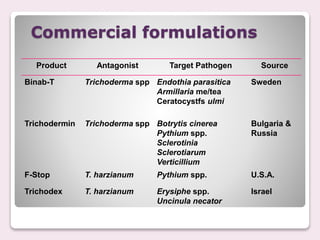
Trichoderma spp. are prevalent fungi in agricultural soils that act as biocontrol agents and plant growth promoters by forming mutualistic relationships with plants and inhibiting soil-borne pathogens such as Pythium and Rhizoctonia. They possess antifungal properties through mechanisms like mycoparasitism and antibiosis, and some commercial strains are modified for enhanced resistance to agricultural chemicals. The document details the genetic characteristics, mode of action, applications in agriculture, and potential industrial uses of Trichoderma, along with examples of various species and commercially available formulations.