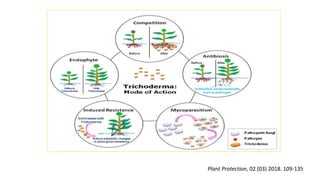
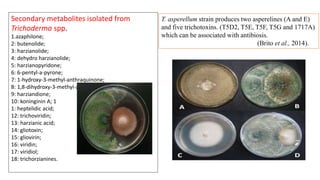
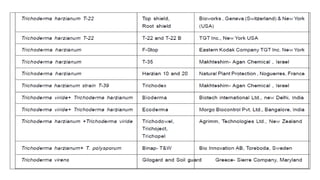
The document discusses Trichoderma, a group of asexually reproducing fungi, highlighting its biological characteristics, methods of pathogen interaction, and its role in plant growth promotion. It elaborates on the mechanisms of mycoparasitism, antibiosis, and competition in plant-pathogen dynamics, as well as the various secondary metabolites produced that contribute to these actions. Additionally, the paper emphasizes the applications of Trichoderma in biocontrol and horticulture, alongside precautions for field application and future prospects for commercial products.