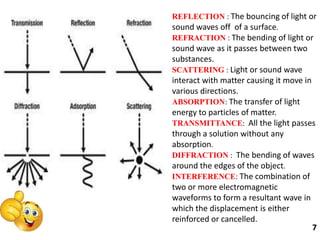
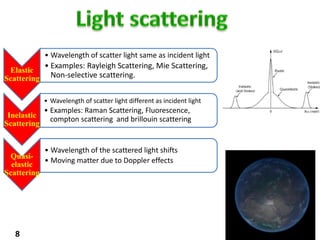
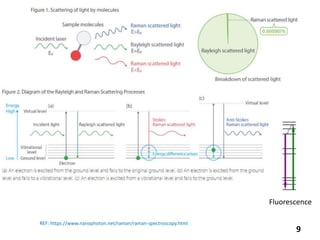
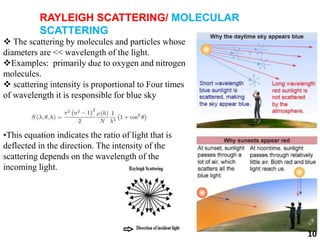
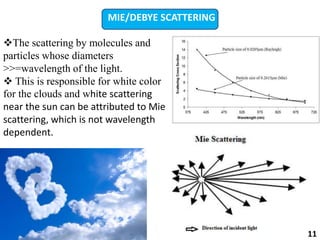
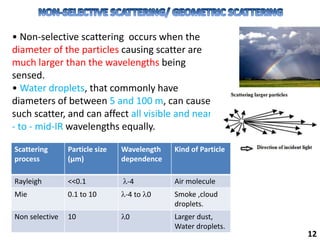
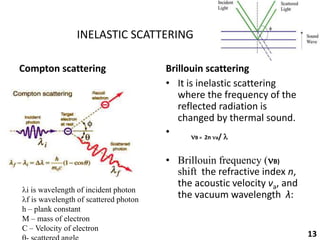
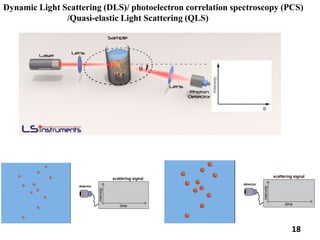
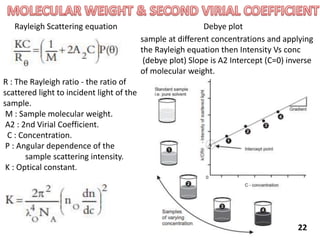
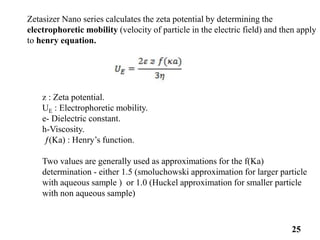
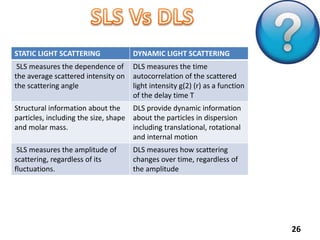
This document provides information about various light scattering techniques used to characterize nanoparticles. It defines key terms like Rayleigh scattering, Mie scattering, dynamic light scattering, and zeta potential. Tables are included that show particle size ranges measured by different techniques like static light scattering, dynamic light scattering, and zeta potential analysis. Fundamental principles are described, such as how dynamic light scattering can measure particle size by analyzing the intensity fluctuations of scattered light. Equations for calculating properties like the second virial coefficient and zeta potential from light scattering measurements are also shown.