Kajal Patel presented on nucleophilic displacement reactions to the M.Sc class. The presentation covered SN1 and SN2 reactions.
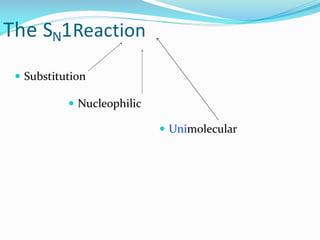
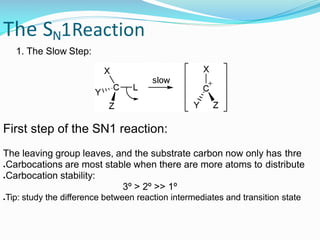
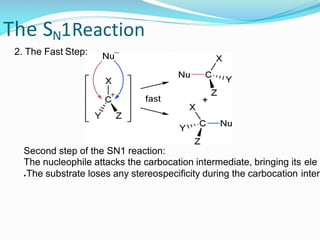
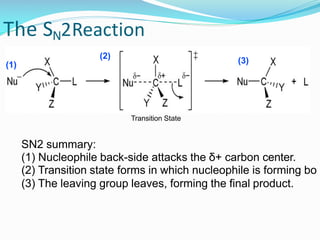
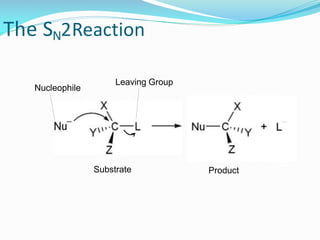
SN1 reactions are nucleophilic substitutions that proceed through a carbocation intermediate. They are unimolecular, depending only on the concentration of one reactant, and lose stereochemistry. SN2 reactions are also nucleophilic substitutions, but are bimolecular and proceed in one step via a backside attack, resulting in inversion of configuration.