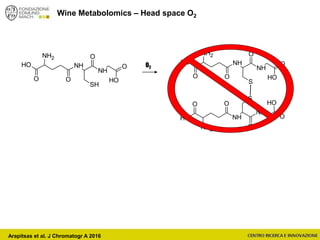
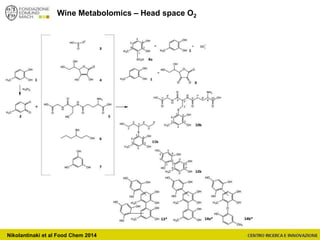
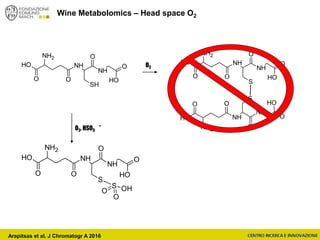
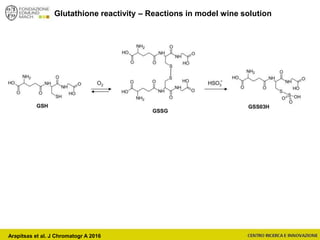
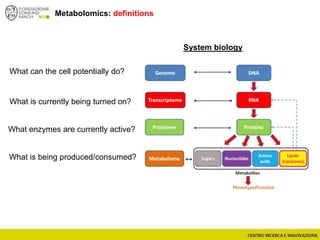
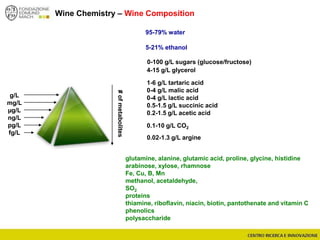
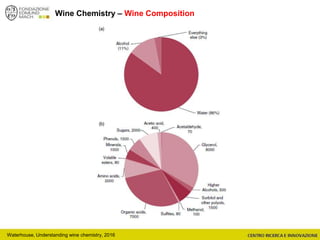
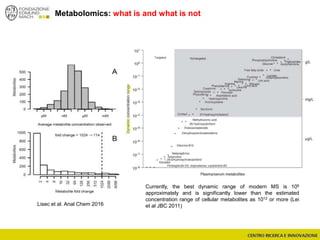
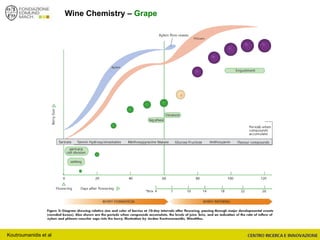
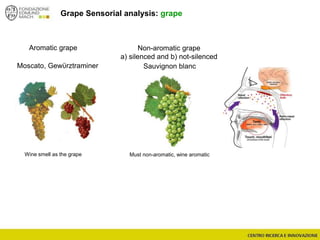
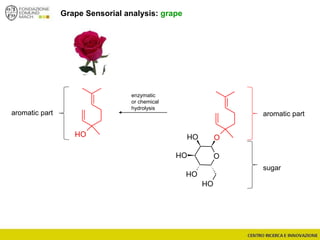
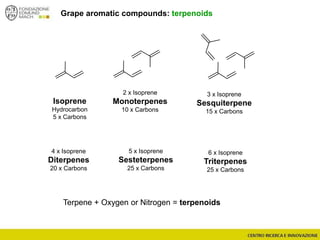
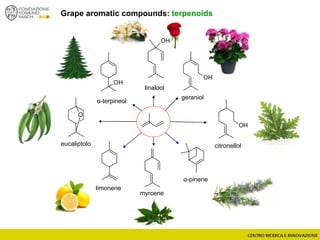
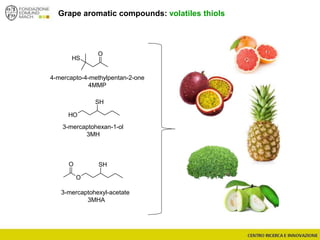
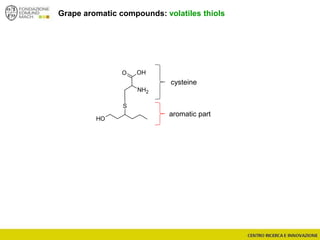
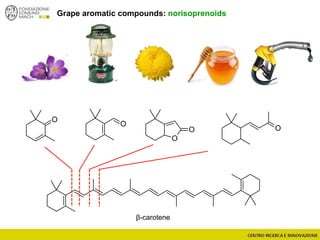
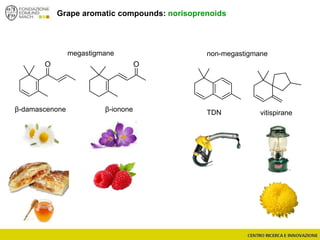
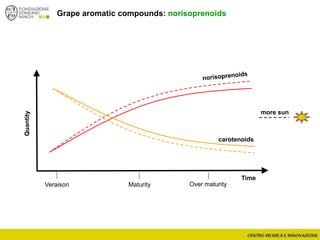
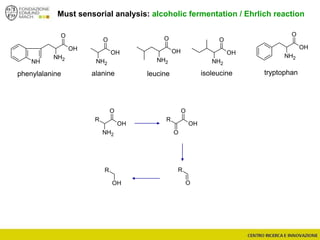
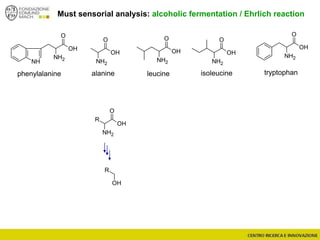
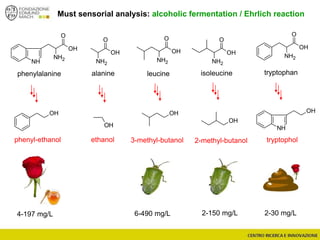
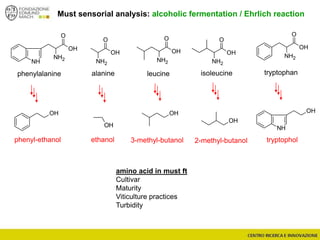
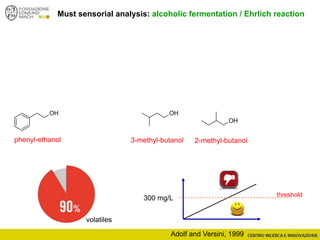
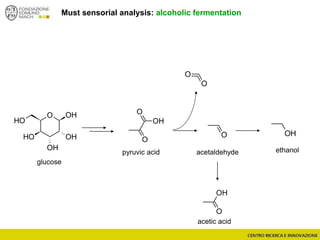
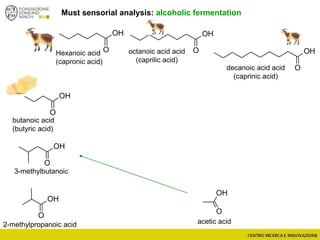
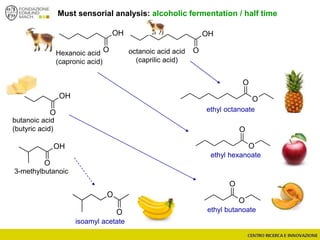
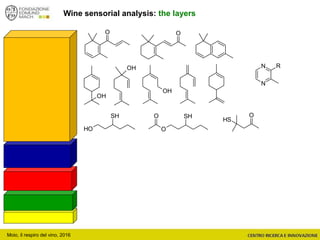
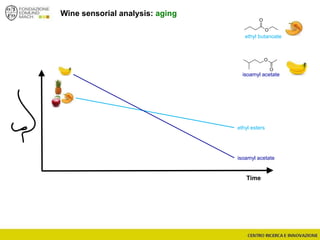
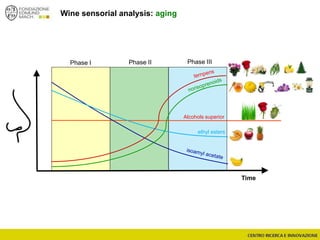
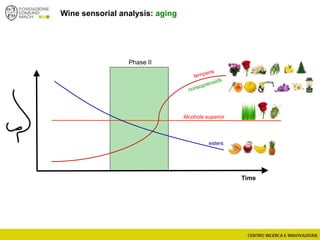
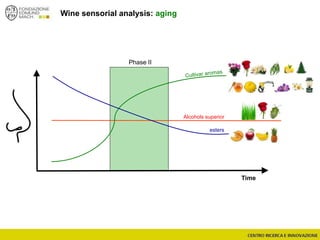
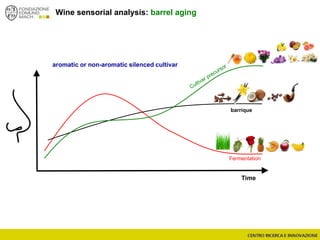
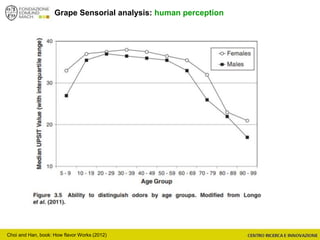
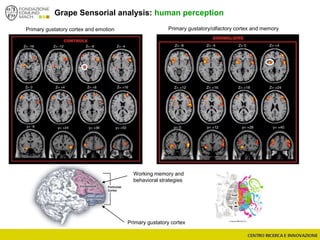
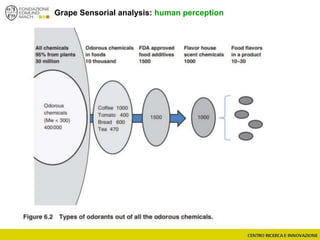
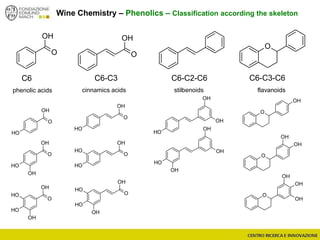
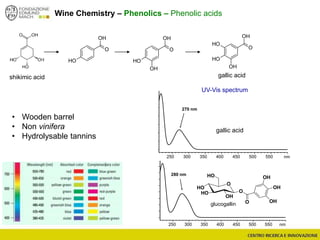
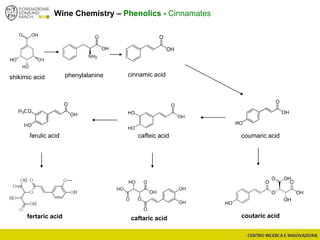
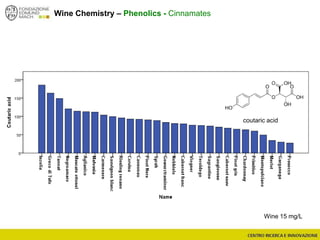
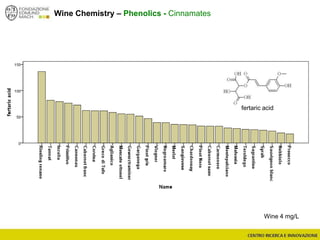
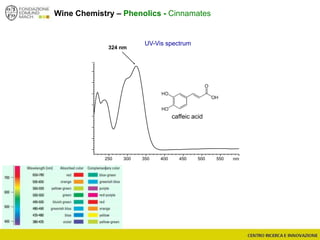
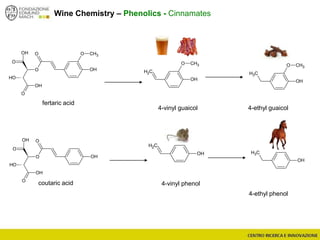
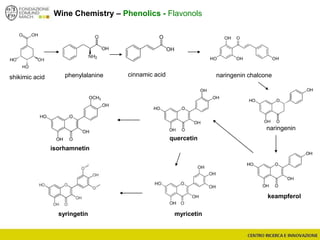
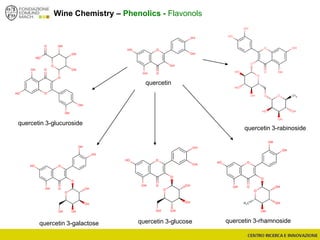
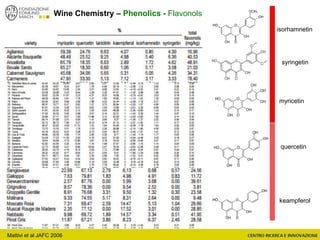
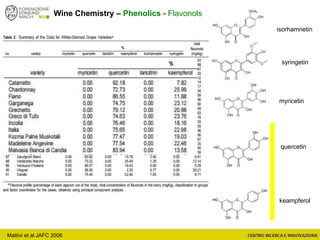
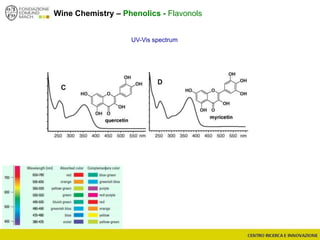
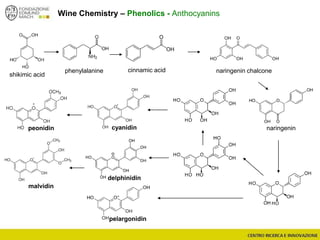
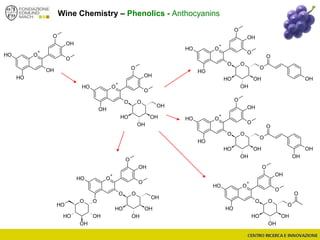
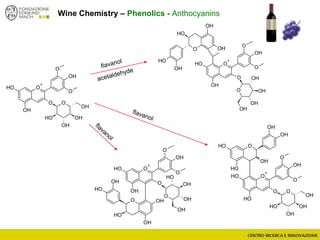
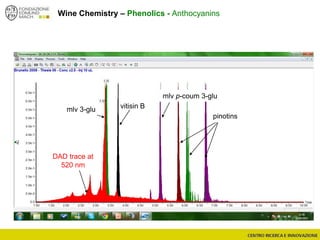
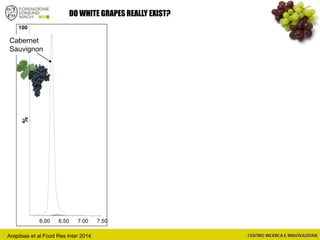
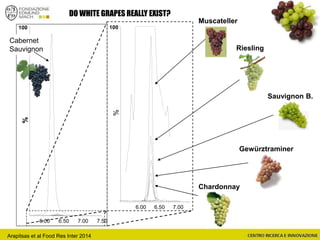
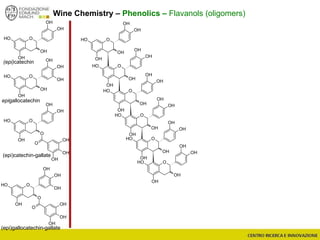
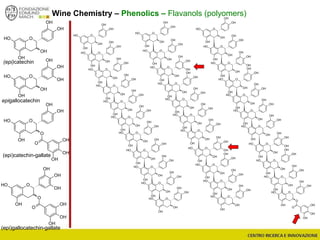
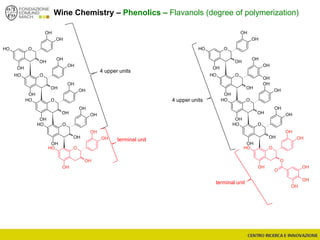
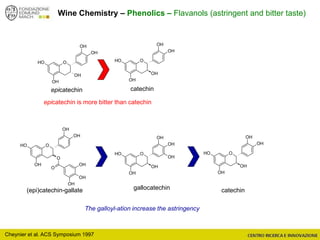
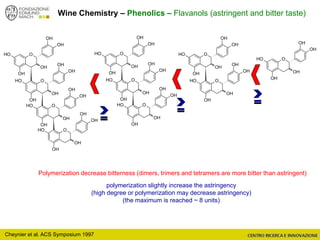
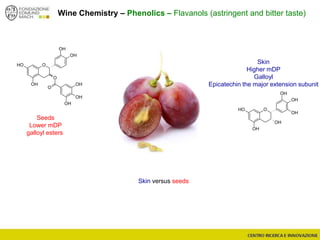
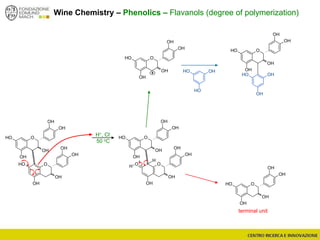
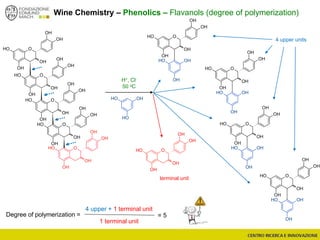
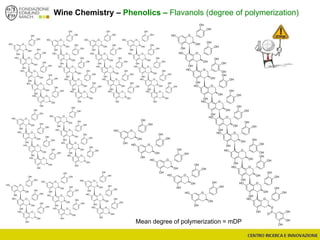
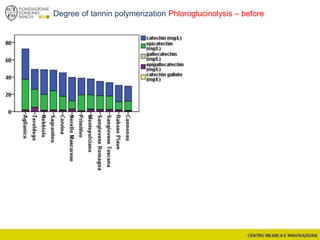
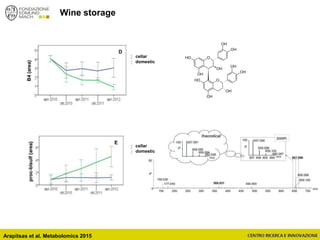
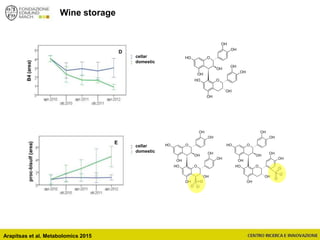
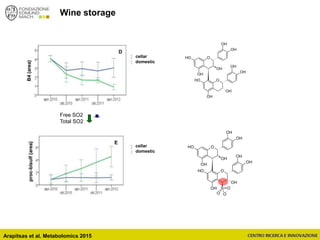
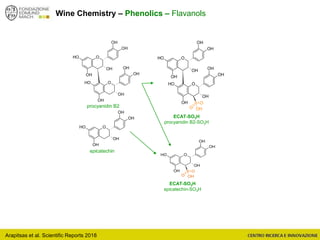
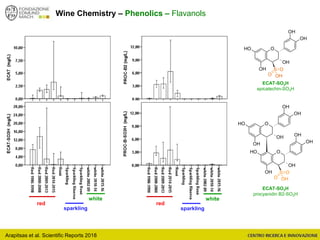
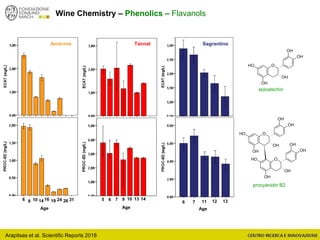
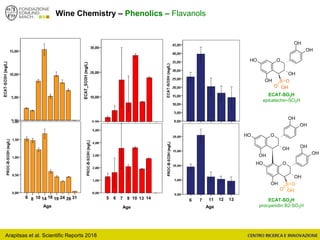
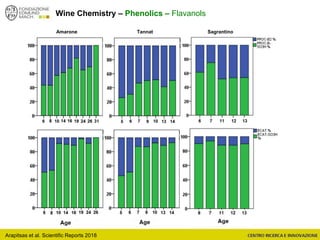
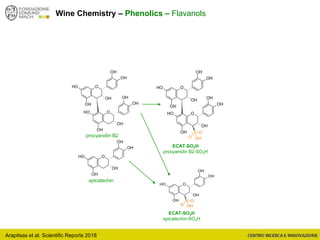
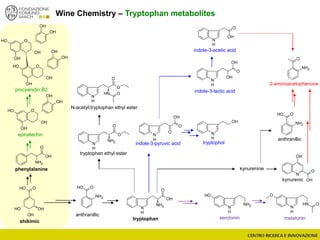
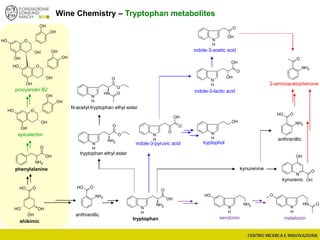
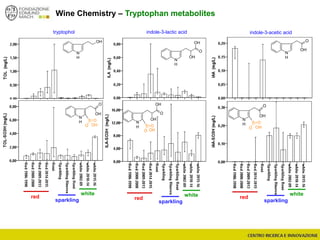
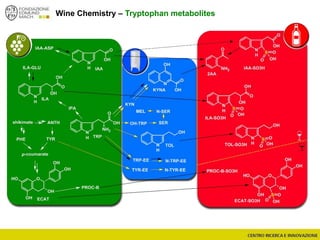
The document discusses metabolomics, outlining its definitions, methods, and the comprehensive study of small-molecule metabolites in biological systems. It provides details on the composition of wine and the significance of grape aromatic compounds in wine chemistry, including classifications of metabolites. Additionally, it touches on grape sensory analysis and human perception of flavors in wine, with an emphasis on the complexities of fermentation and aging processes.


















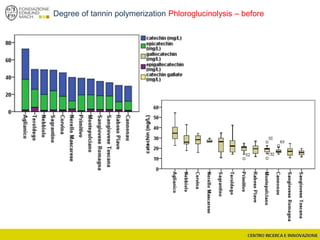
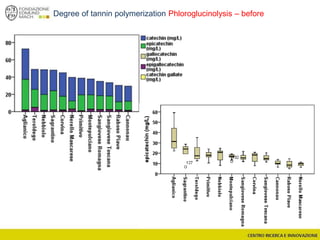
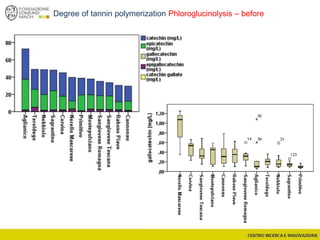
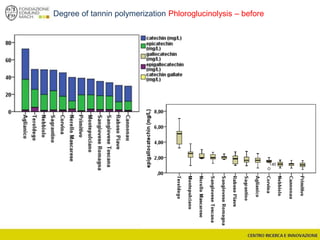



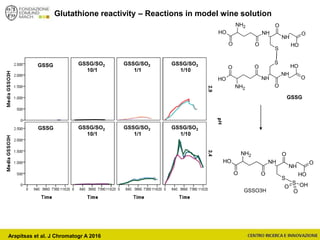
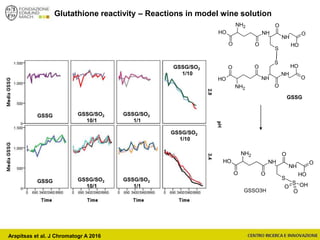
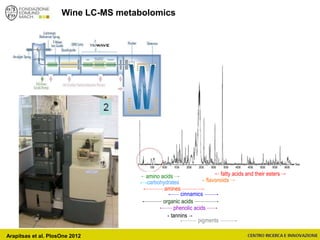
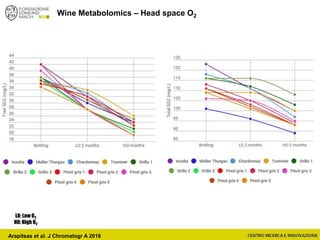
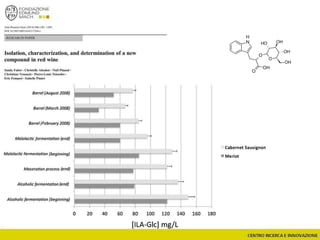
![Wine Metabolomics – Head space O2
-1200
-1100
-1000
-900
-800
-700
-600
-500
-400
-300
-200
-100
0
100
200
300
400
500
600
700
800
900
1000
1100
1200
-600 -500 -400 -300 -200 -100 0 100 200 300 400 500 600
t[1]
t[4]
Scores Comp[4] vs. Comp[1] colored by Variety
C
G
In
M
P
Q
T
Hotelling’s T2 Ellipse (95%) = (559.1; 1139)
R2X[4] = 0.06998
R2X[1] = 0.2906
EZinf o 2 - nomacorc2_1QC (M3: PCA-X) - 2015-01-23 12:22:22 (UTC+1)
3 x Grillo
5 x Pinot gris
1 x Inzolia
1 x Muller Thurgau
1 x Chardonnay
1 x Traminer
6 x varieties
12 x wines
N2 O2
2 x bottling conditions
2 months storage
(4+4)x12 = 96 bottles (+)
~8000 features
QC
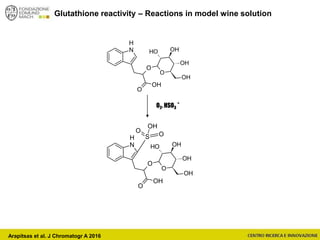
Arapitsas et al. J Chromatogr A 2016](https://image.slidesharecdn.com/teimay2018-180607075305/85/Wine-Chemistry-152-320.jpg)


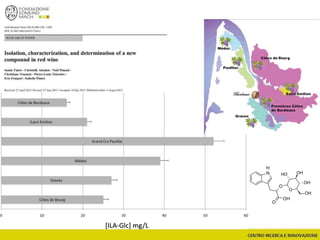
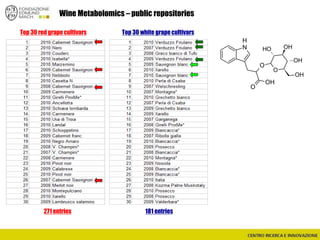
![Wine Metabolomics – Head space O2
-1200
-1100
-1000
-900
-800
-700
-600
-500
-400
-300
-200
-100
0
100
200
300
400
500
600
700
800
900
1000
1100
1200
-600 -500 -400 -300 -200 -100 0 100 200 300 400 500 600
t[1]
t[4]
Scores Comp[4] vs. Comp[1] colored by Variety
C
G
In
M
P
Q
T
Hotelling’s T2 Ellipse (95%) = (559.1; 1139)
R2X[4] = 0.06998
R2X[1] = 0.2906
EZinf o 2 - nomacorc2_1QC (M3: PCA-X) - 2015-01-23 12:22:22 (UTC+1)
3 x Grillo
5 x Pinot gris
1 x Inzolia
1 x Muller Thurgau
1 x Chardonnay
1 x Traminer
6 x varieties
12 x wines
N2 O2
2 x bottling conditions
2 months storage
(4+4)x12 = 96 bottles (+)
~8000 features
QC
Arapitsas et al. J Chromatogr A 2016](https://image.slidesharecdn.com/teimay2018-180607075305/85/Wine-Chemistry-166-320.jpg)