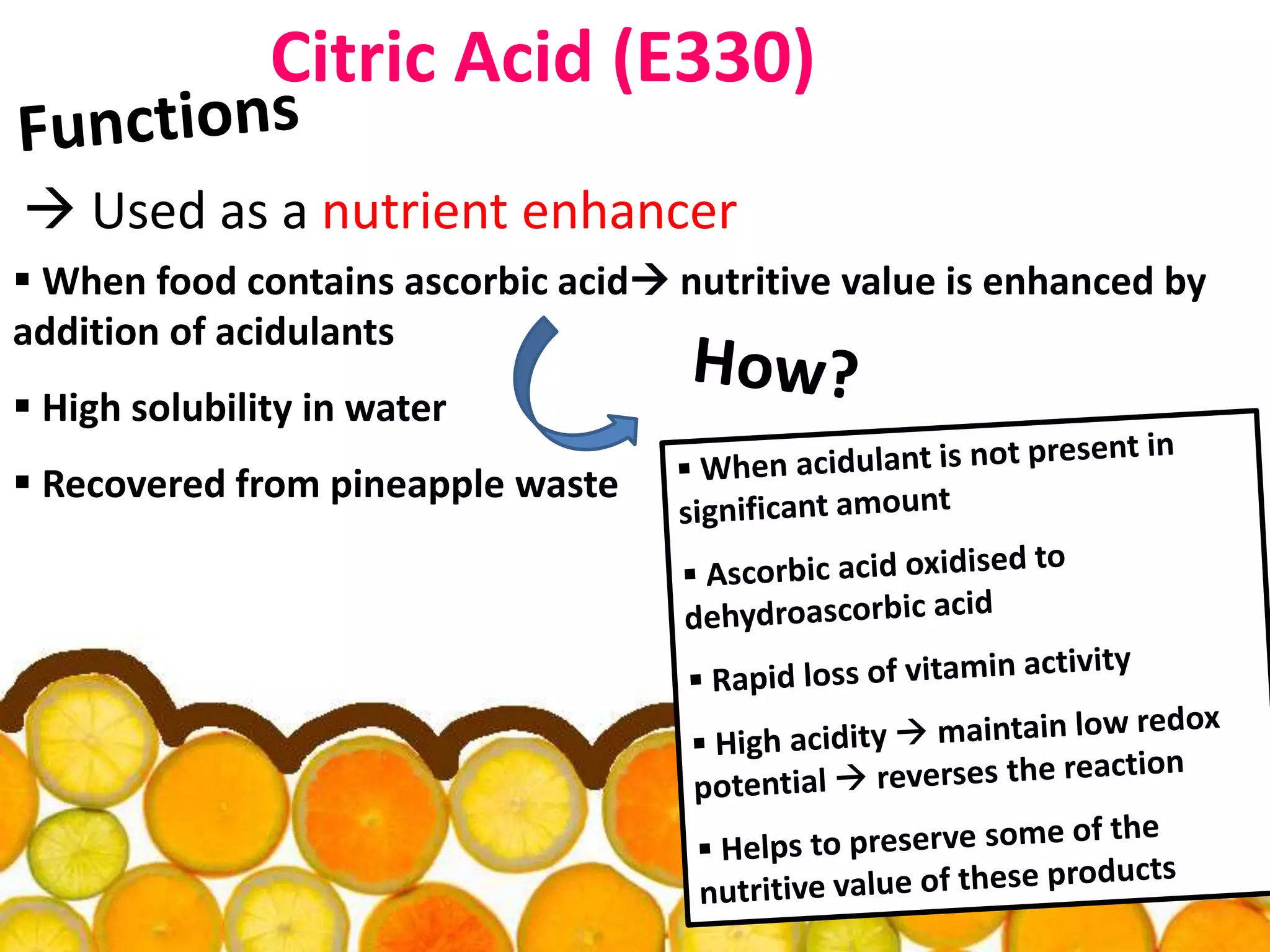
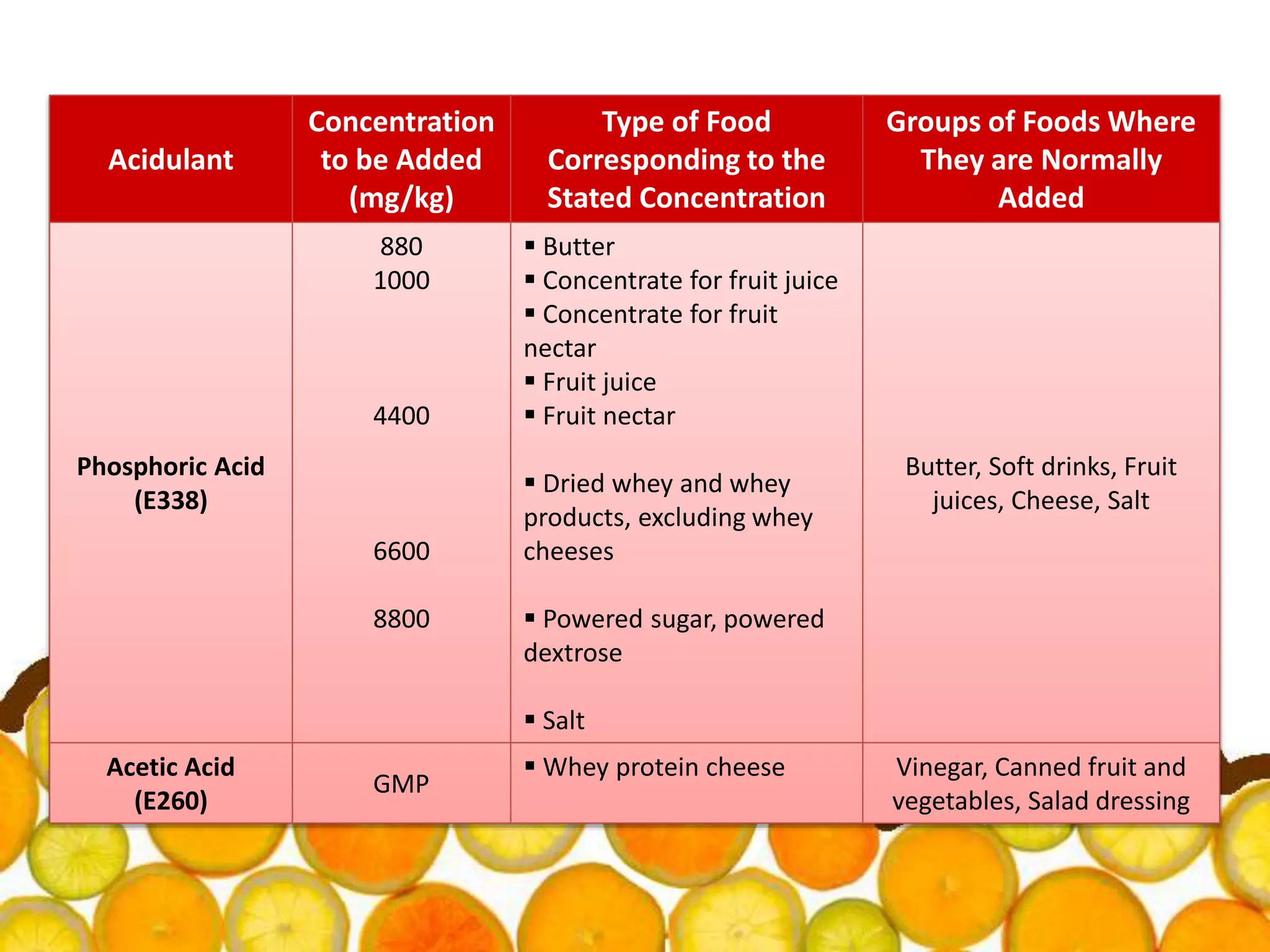
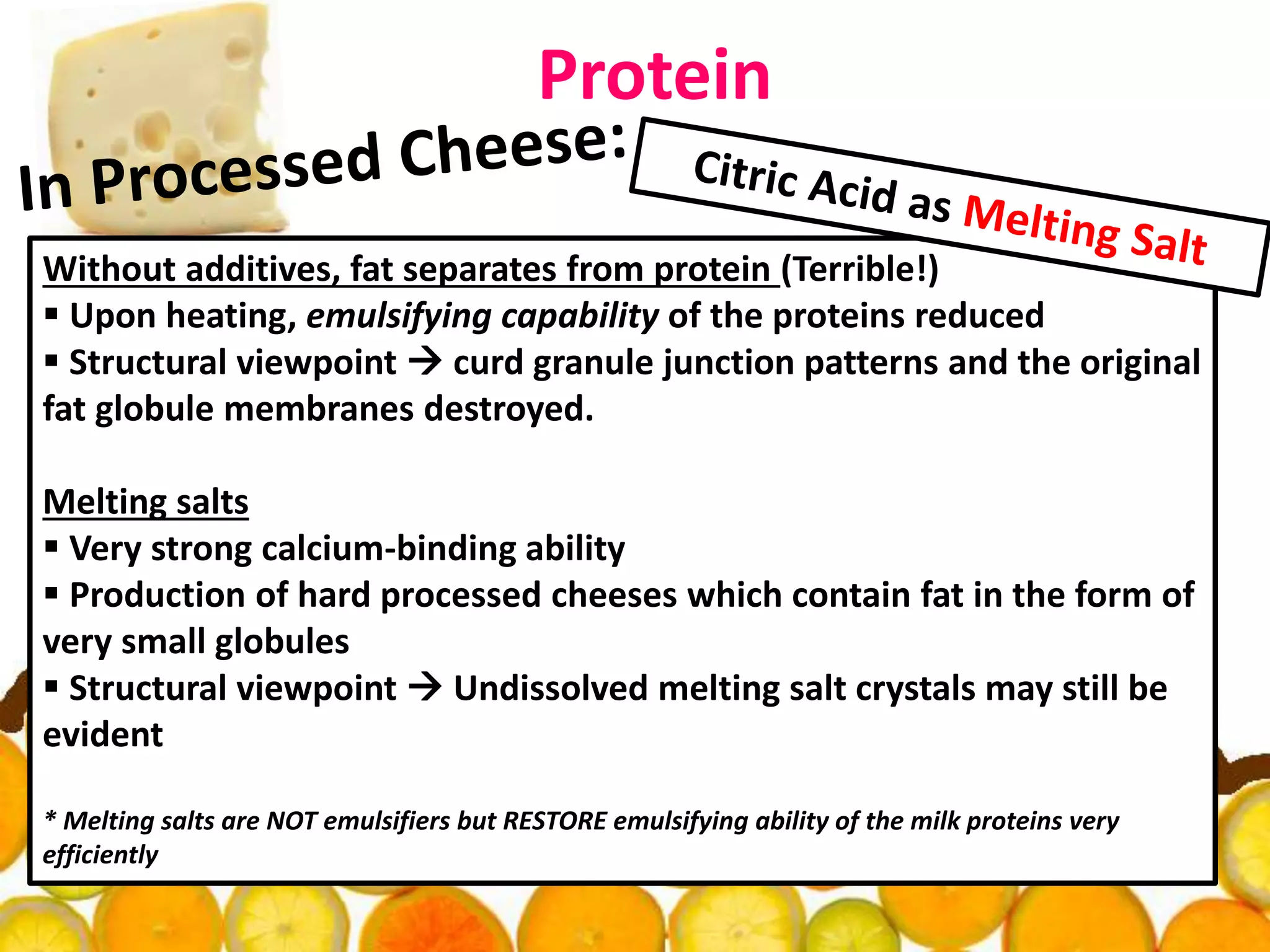
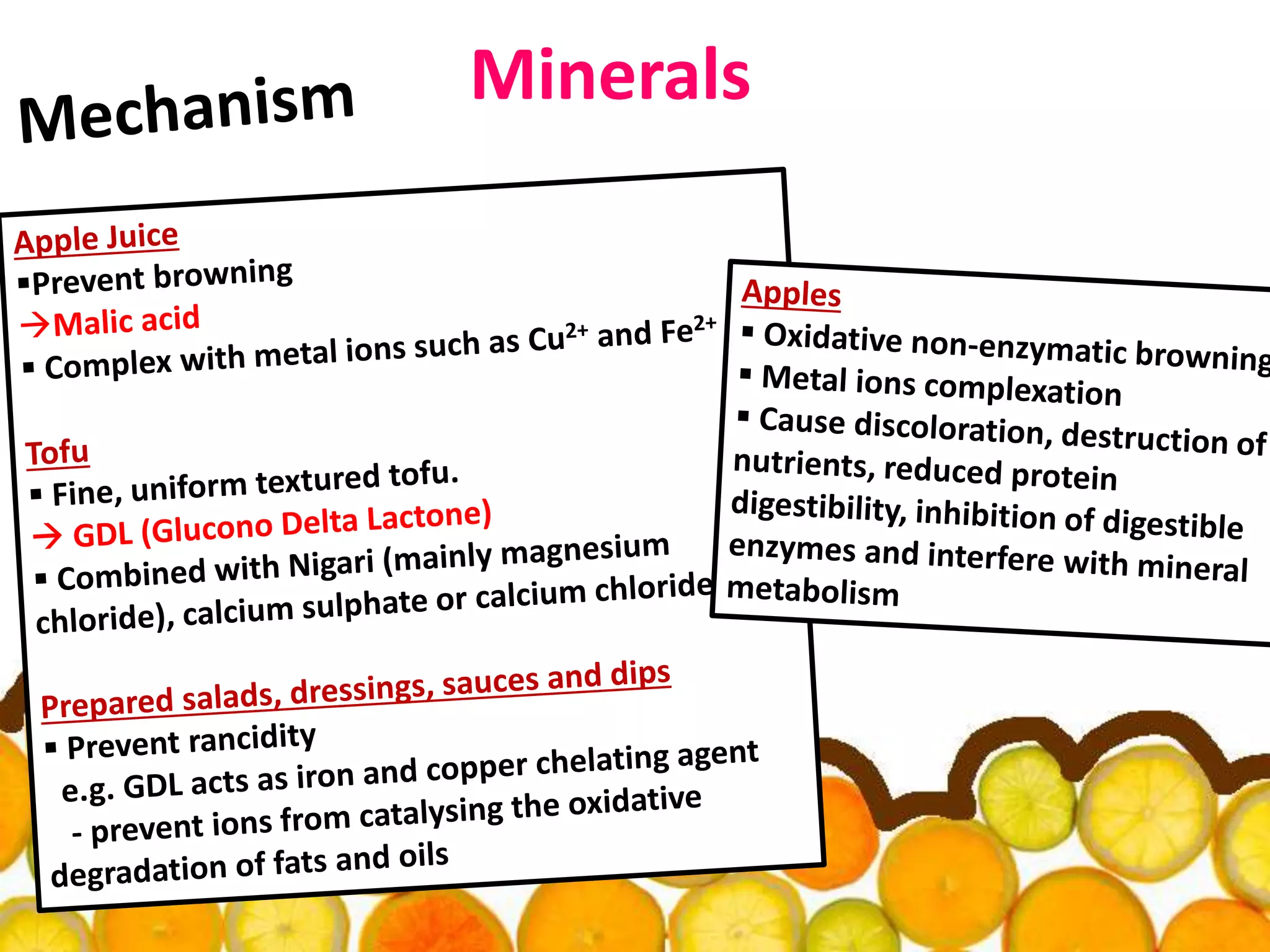
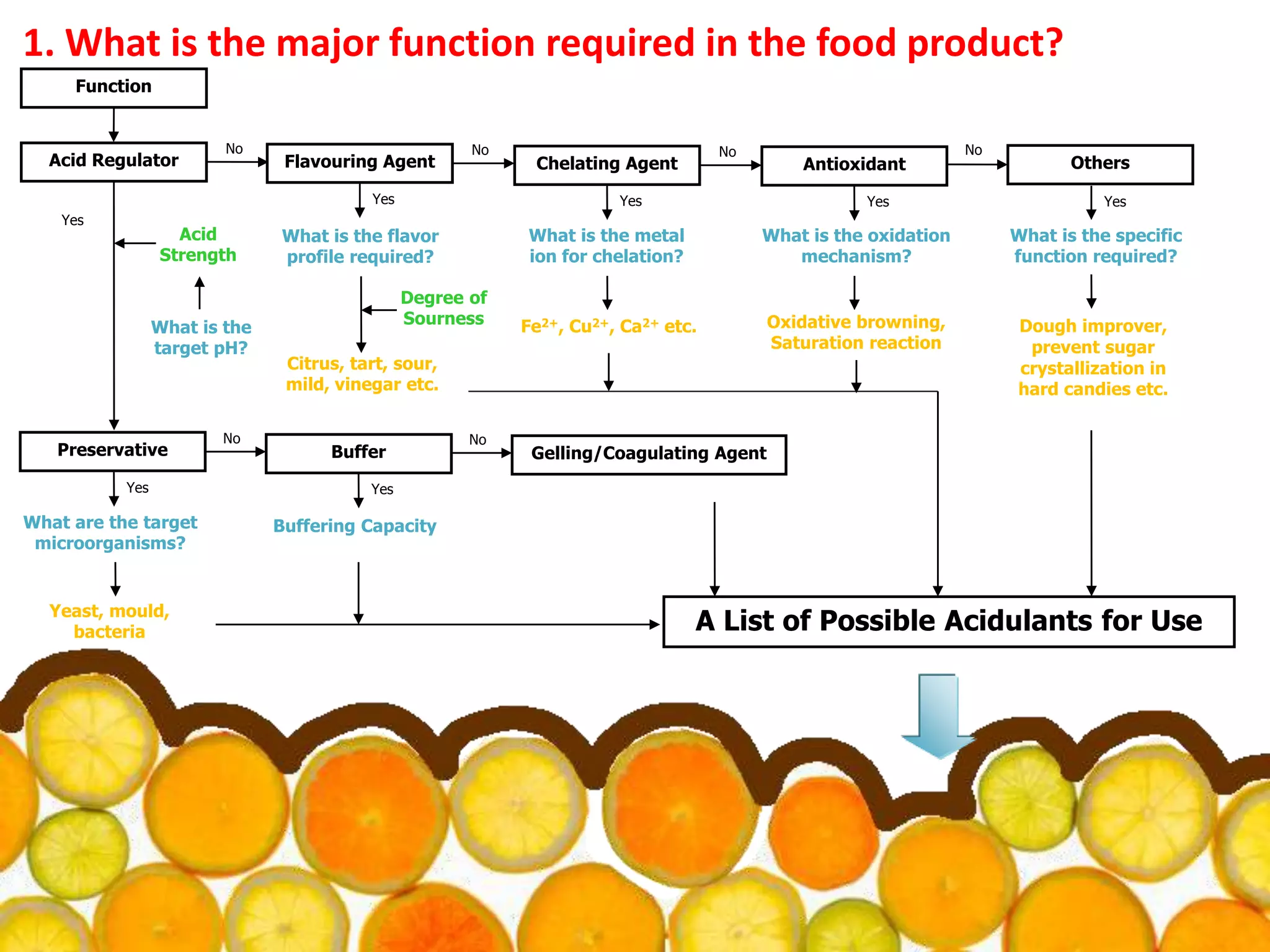
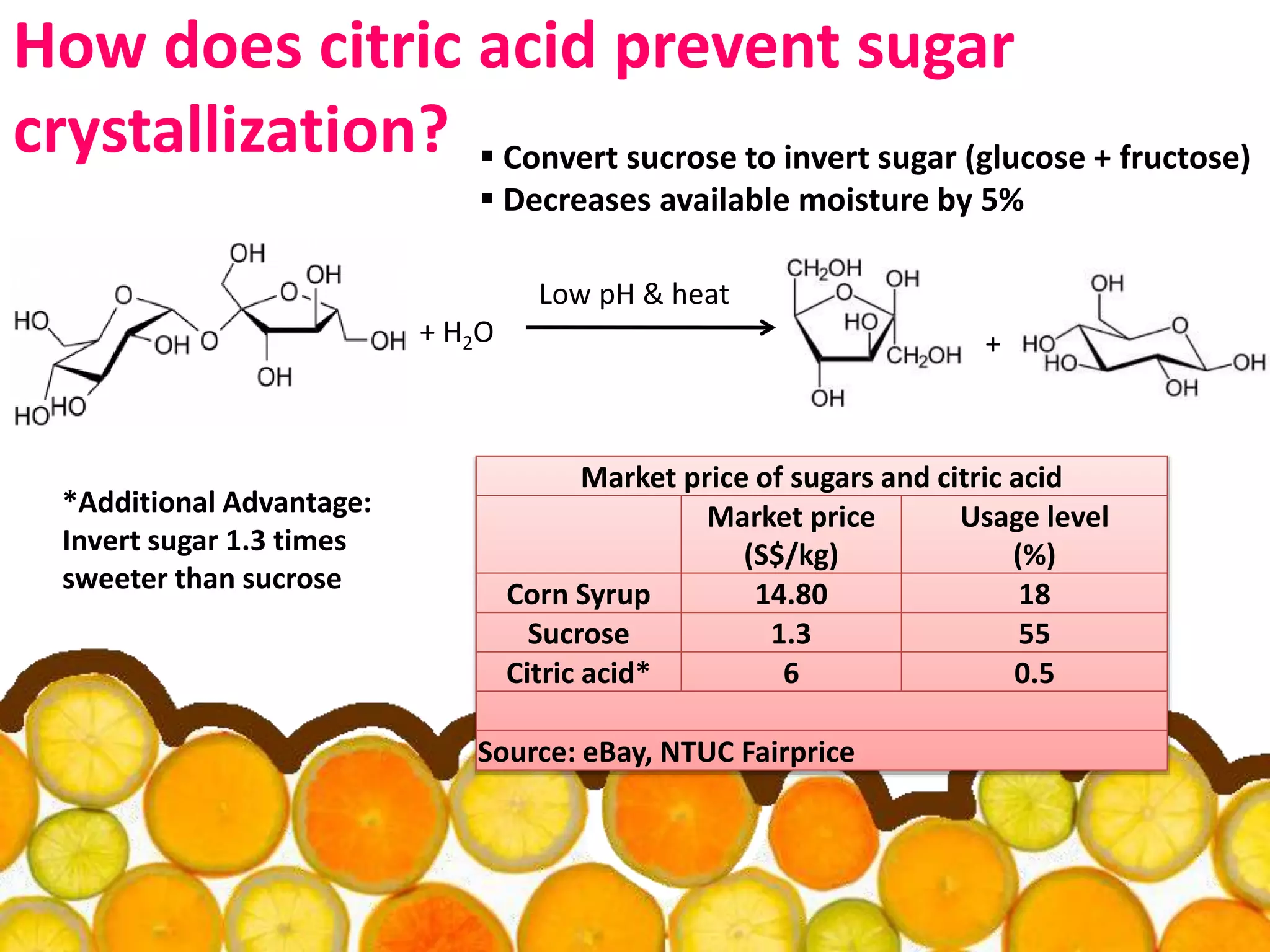
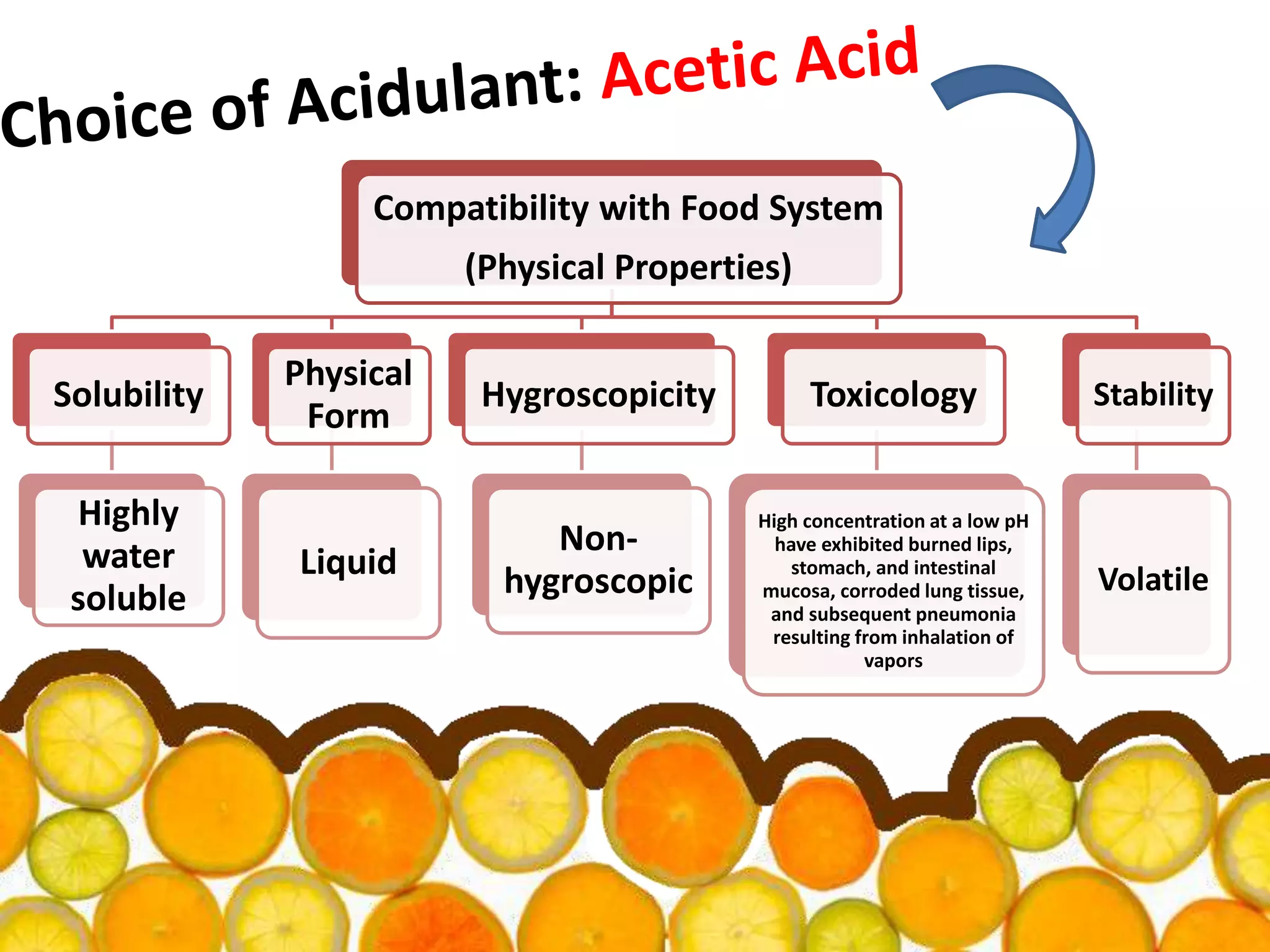
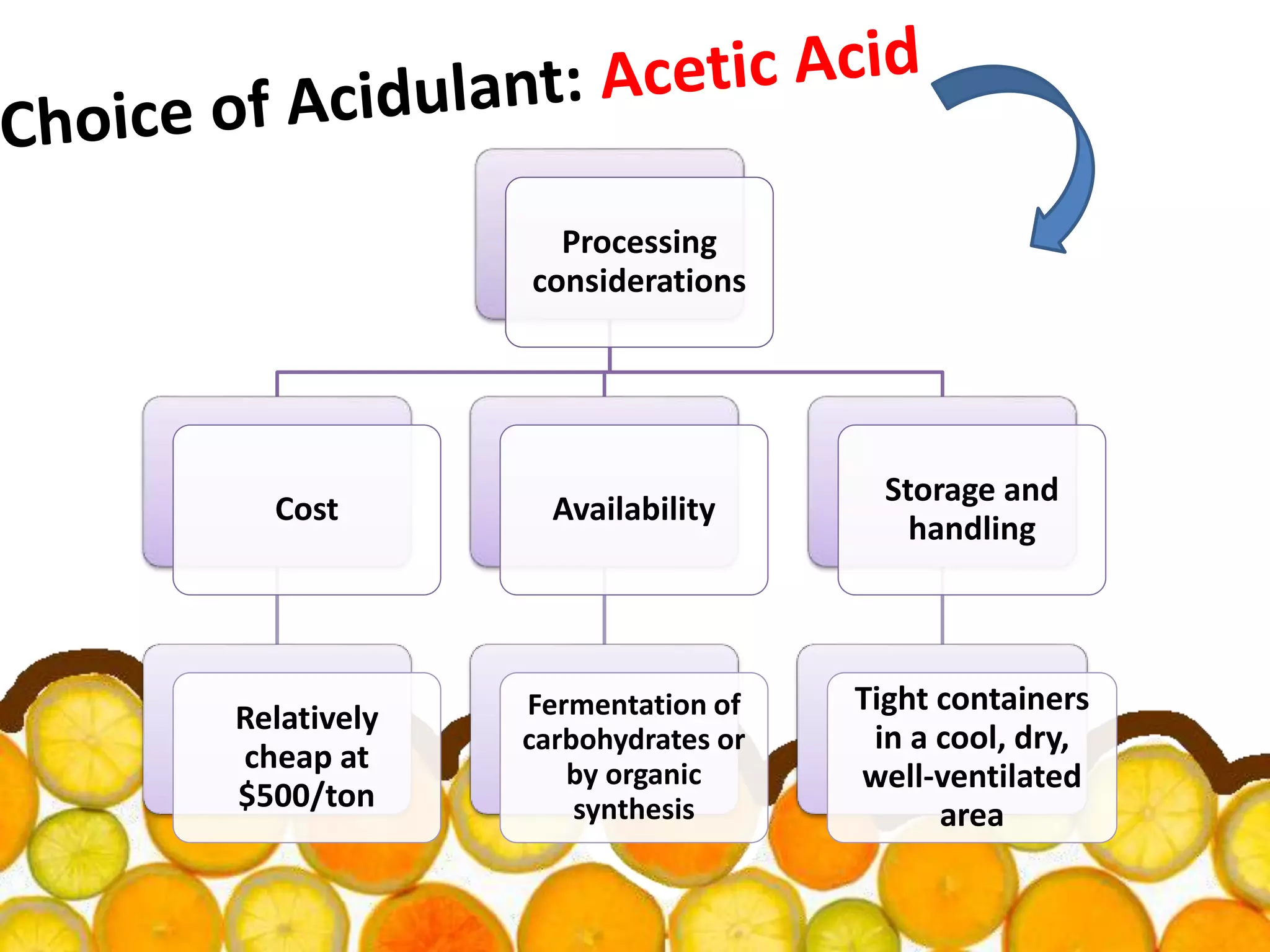
Acidulants are food additives that are used to regulate acidity or pH levels in foods. Common acidulants include citric acid, acetic acid, phosphoric acid, and tartaric acid. The document discusses the functions and uses of various acidulants in foods. It provides information on how acidulants interact with other food constituents like proteins, lipids, carbohydrates, and vitamins. Guidelines are presented on selecting the appropriate acidulant based on the major function required, compatibility with the food system, processing considerations, and legal requirements.