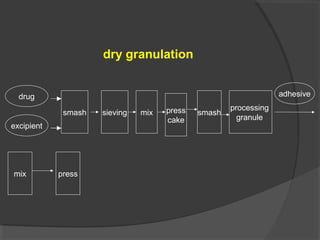
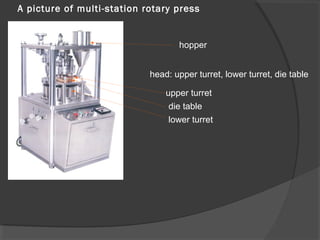
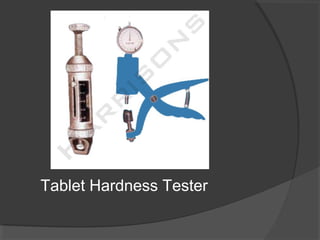
Tablets are solid dosage forms of medication prepared by compressing drugs, available in various forms such as compressed, delayed-release, and chewable tablets. While they offer advantages like stability and cost-effectiveness, they can pose challenges in swallowing and formulation for certain drugs. The manufacturing and formulation of tablets involve various excipients that impact drug release and bioavailability, and quality standards dictate their physical characteristics and performance.