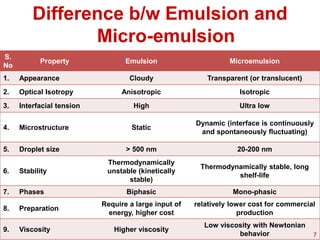
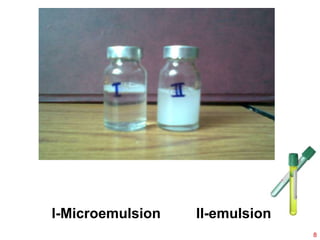
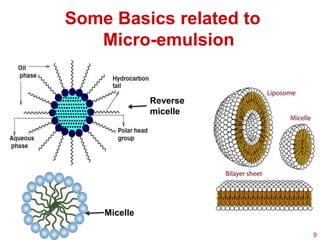
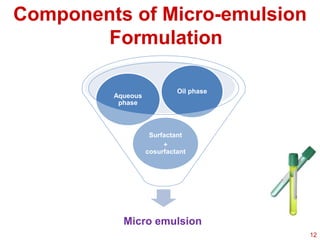
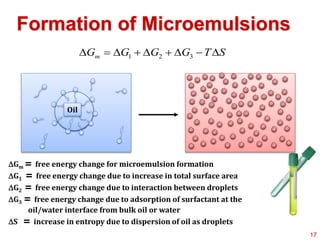
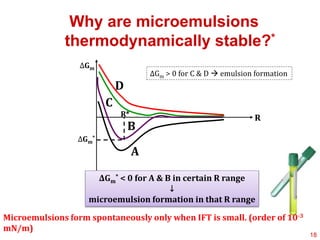
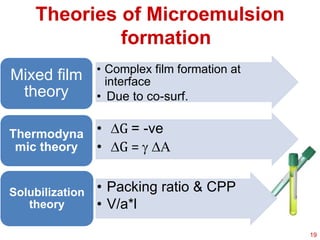
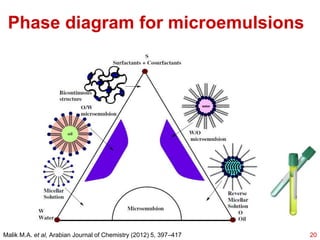
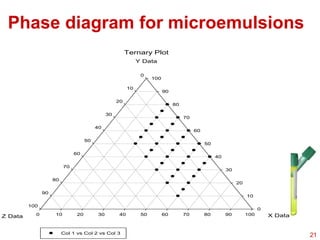
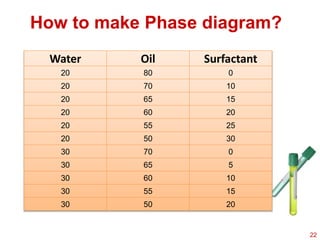
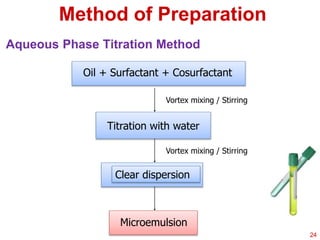
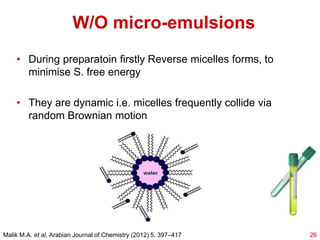
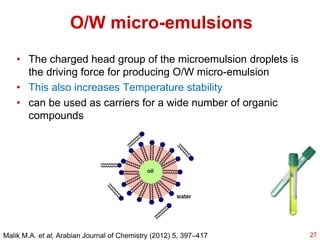
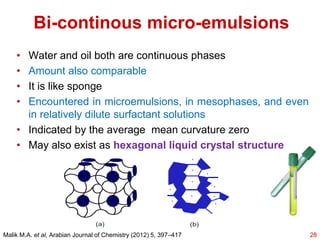
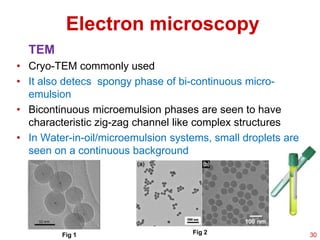
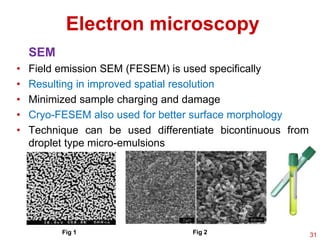
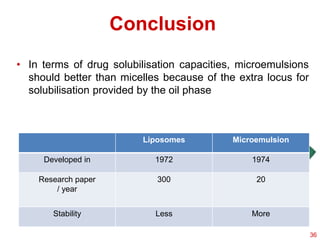
This document provides an overview of microemulsions, including their definition, history, and significance in drug delivery systems. It discusses the properties distinguishing microemulsions from traditional emulsions, their components, formation methods, and characterization techniques. Additionally, the document highlights recent advancements and applications, such as enhanced drug solubilization and stability in pharmaceutical formulations.