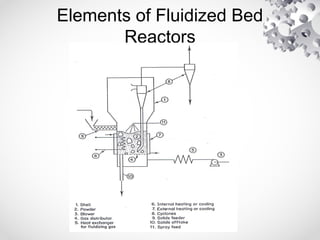
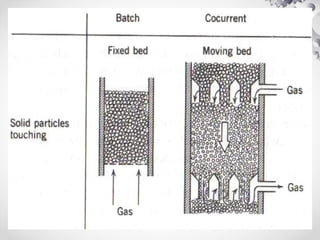
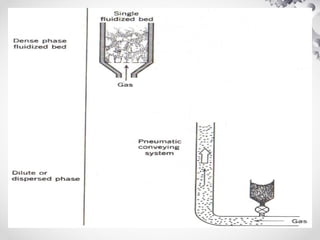
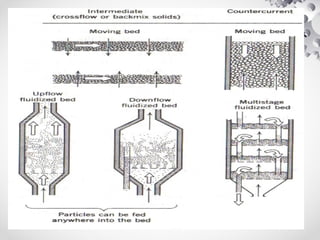
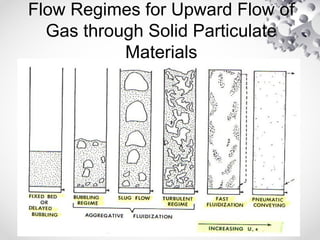
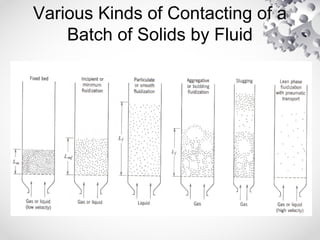
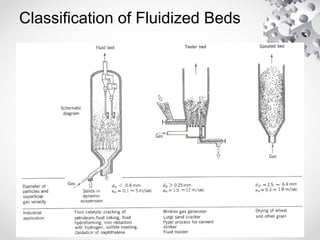
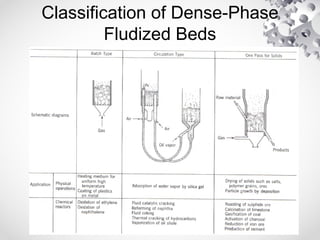
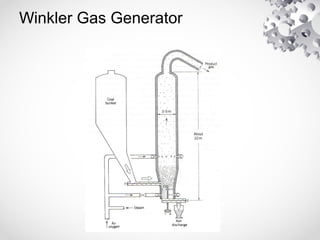
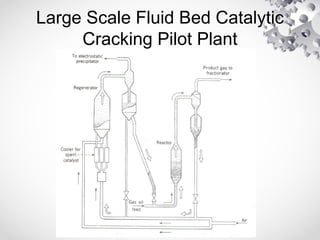
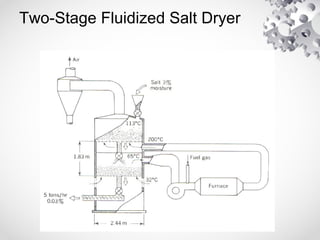
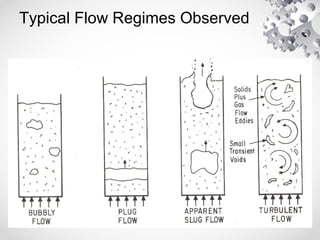
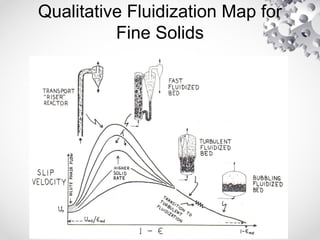
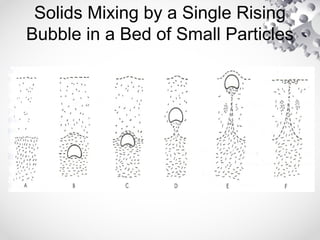
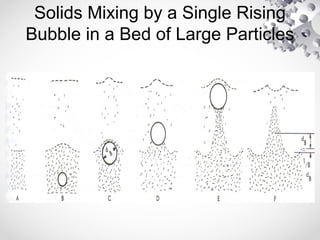
The document discusses fluidization, the process of converting fine solids into a fluid-like state using gas or liquid. It outlines key terminology related to fluidized beds, advantages, disadvantages, and various commercial applications, including solid-catalyzed reactions and drying processes. Additionally, it describes different flow regimes and classifications of fluidized beds relevant to industrial processes.