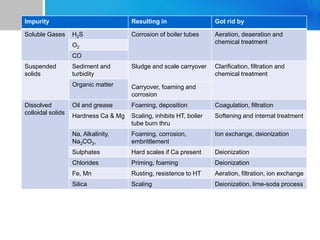
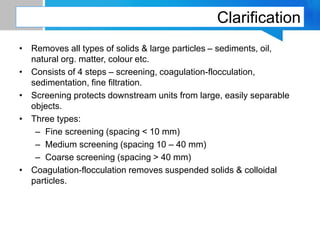
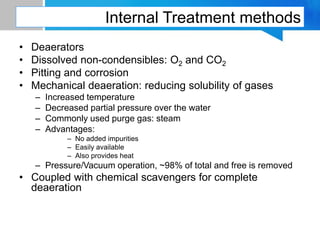
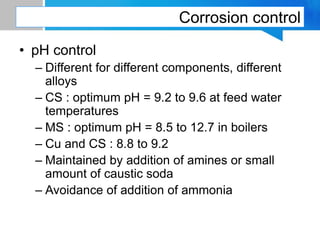
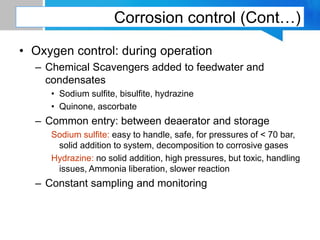
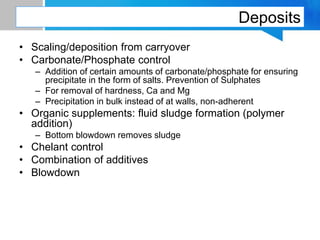
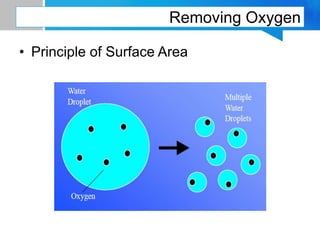
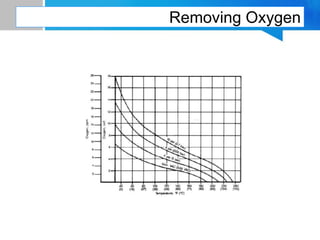
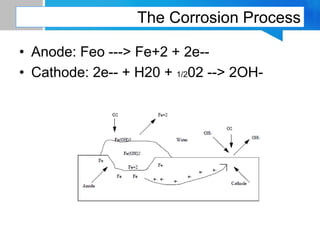
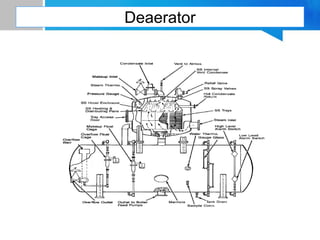
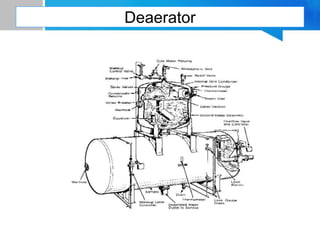
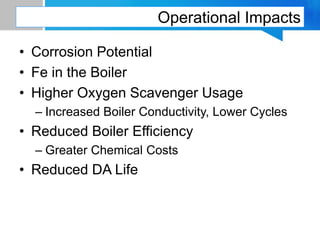
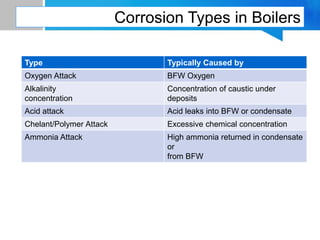
Boiler feed water requires treatment to remove various contaminants that can cause issues like corrosion, scaling, and deposition. There are external and internal treatment methods. External methods include clarification, filtration, ion exchange, and membrane separation techniques like reverse osmosis to remove suspended solids, dissolved salts, and other impurities. Internal methods include deaeration to remove oxygen and carbon dioxide, pH control using amines or caustic soda, and chemical addition for corrosion and deposit control. Proper deaeration is critical to minimize oxygen and achieve the low ppb levels needed to prevent corrosion, requiring adequate steam flow, venting, and monitoring of deaerator performance.