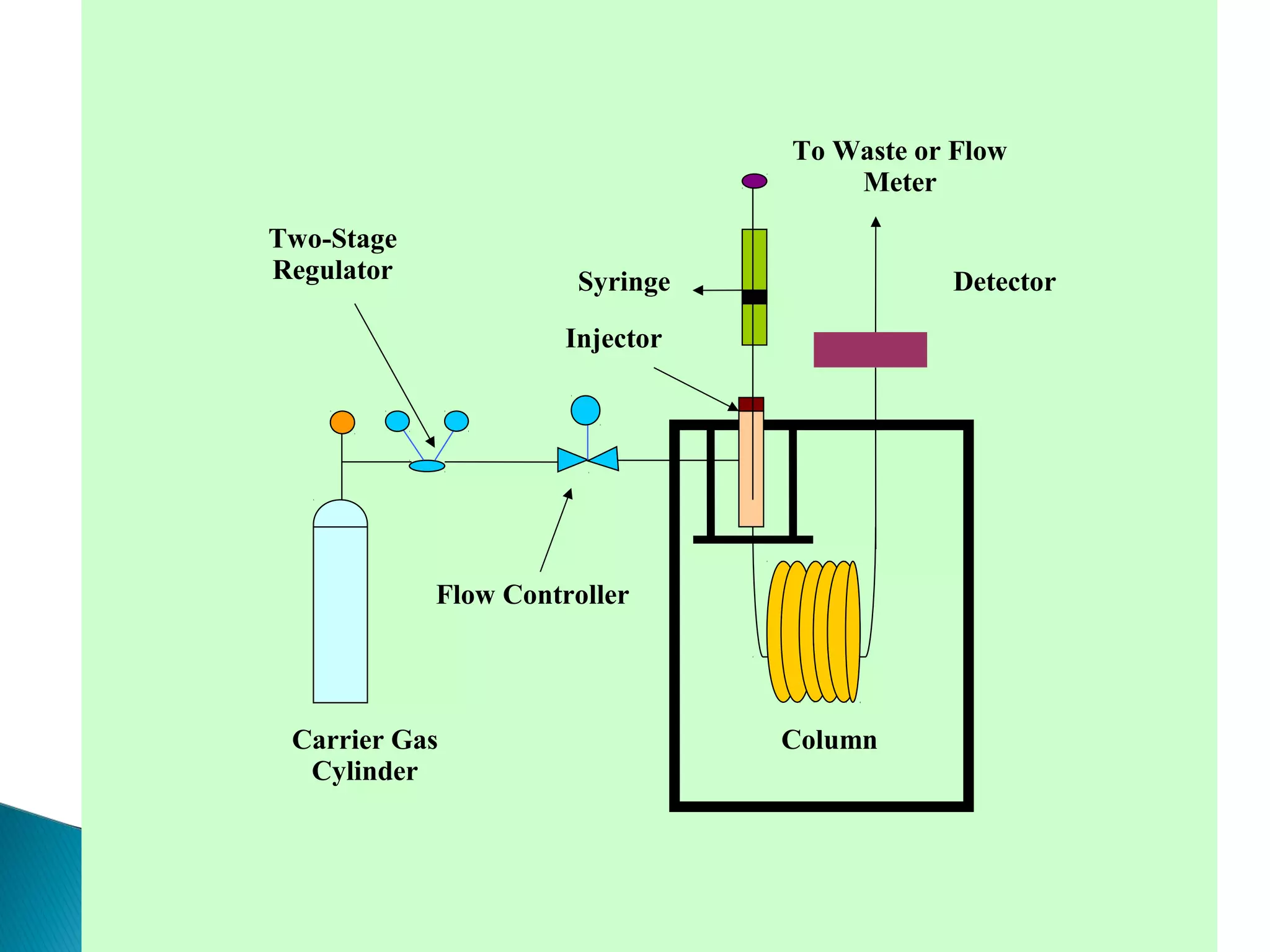
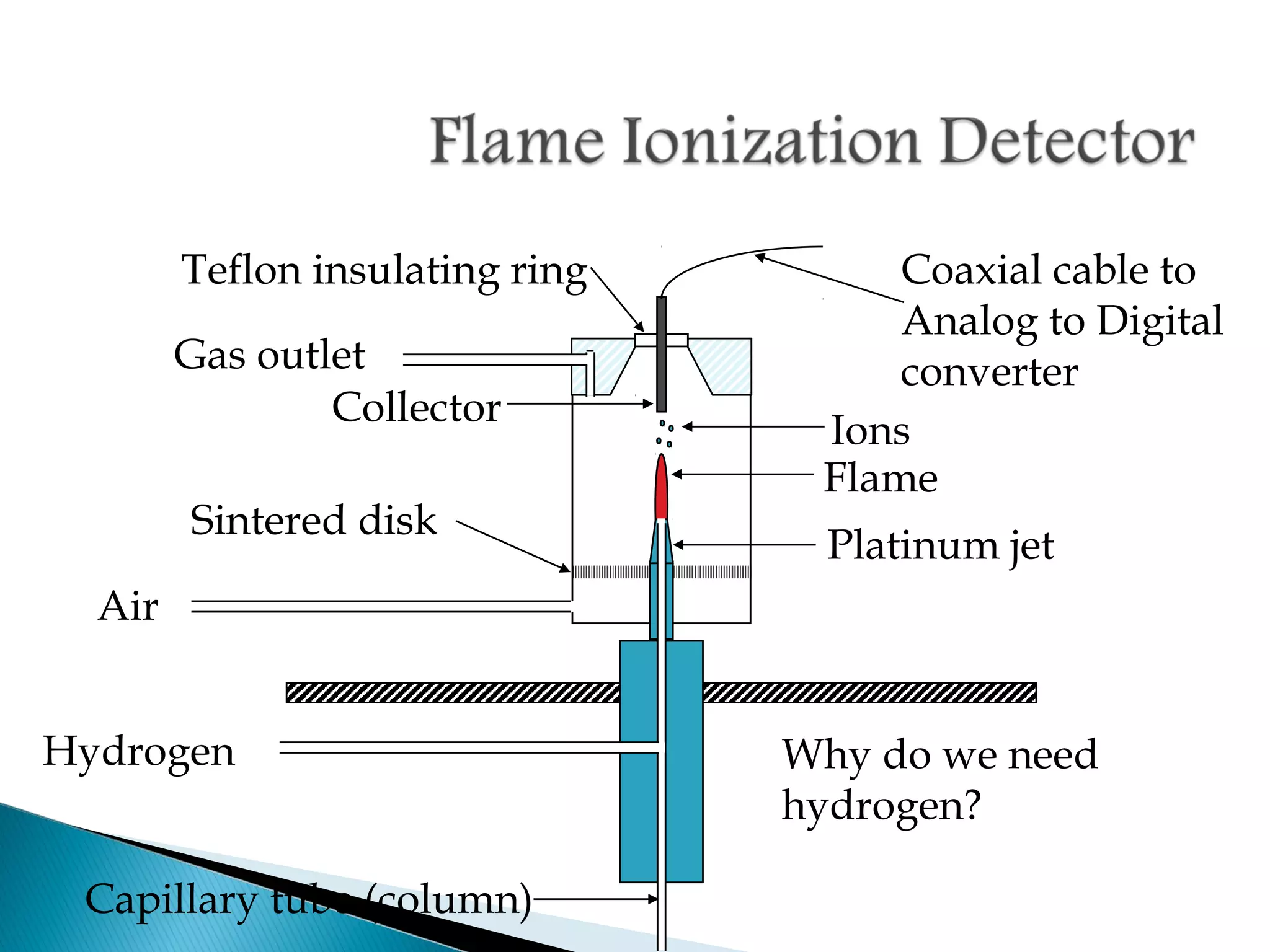
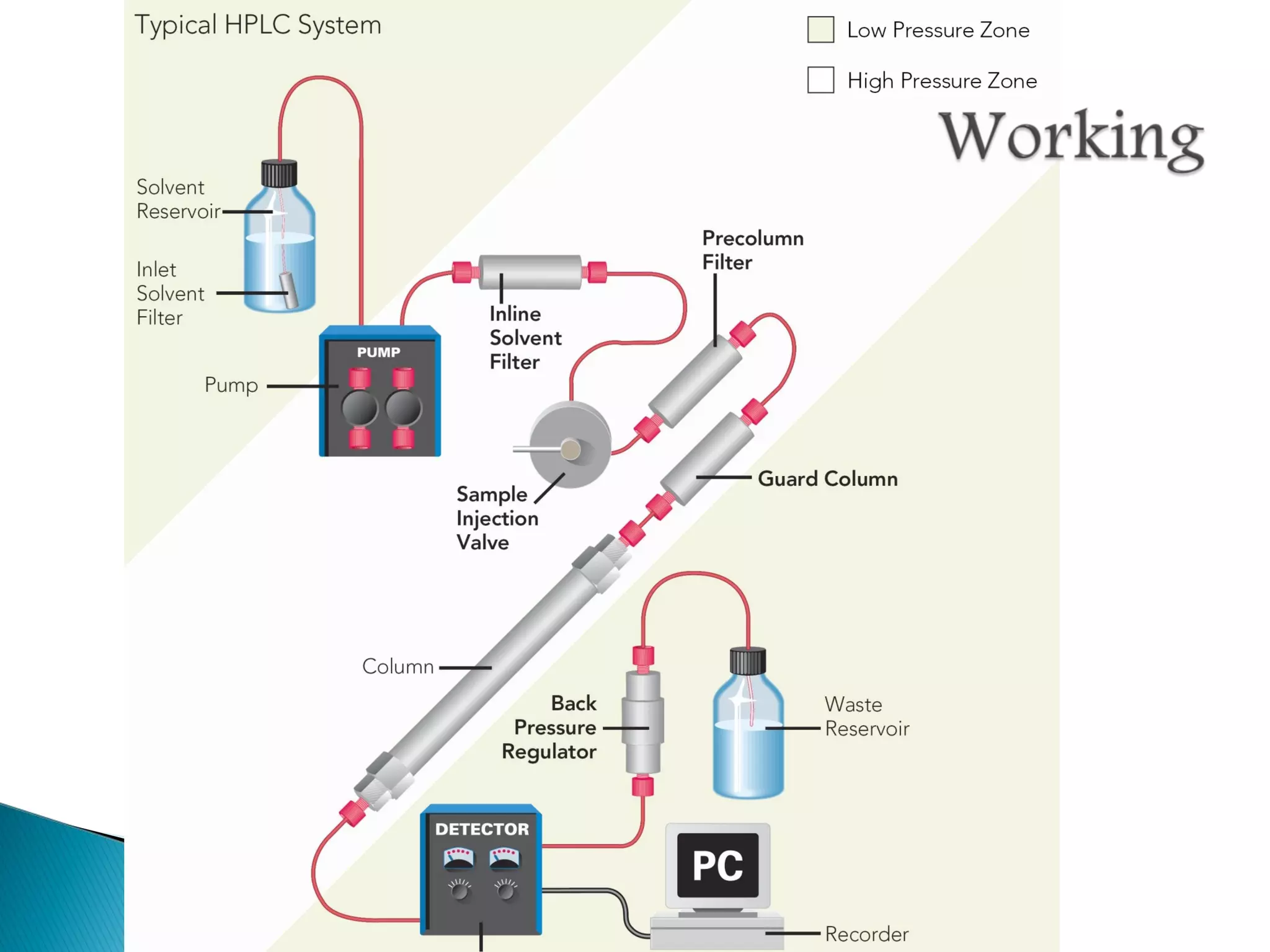
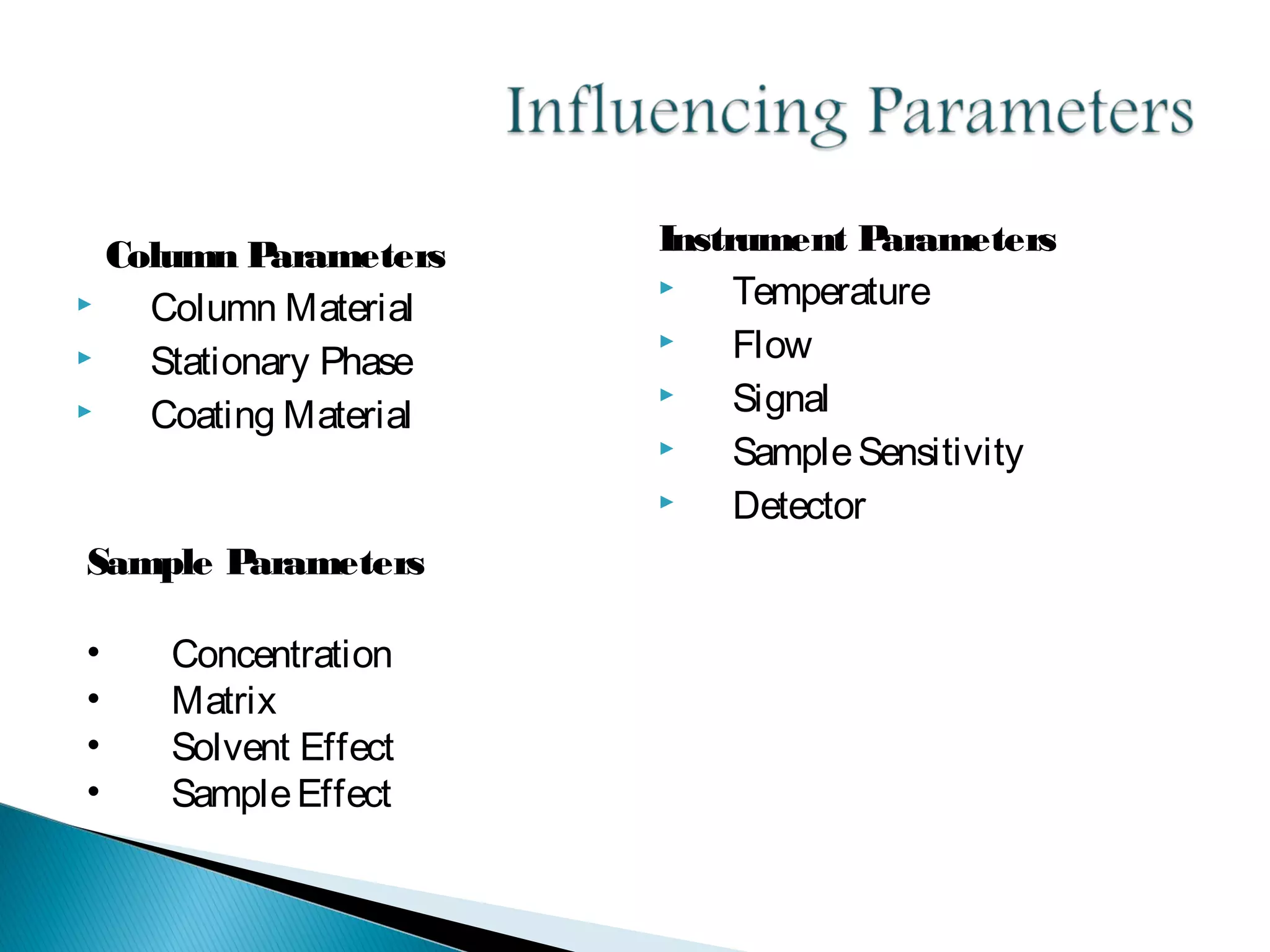
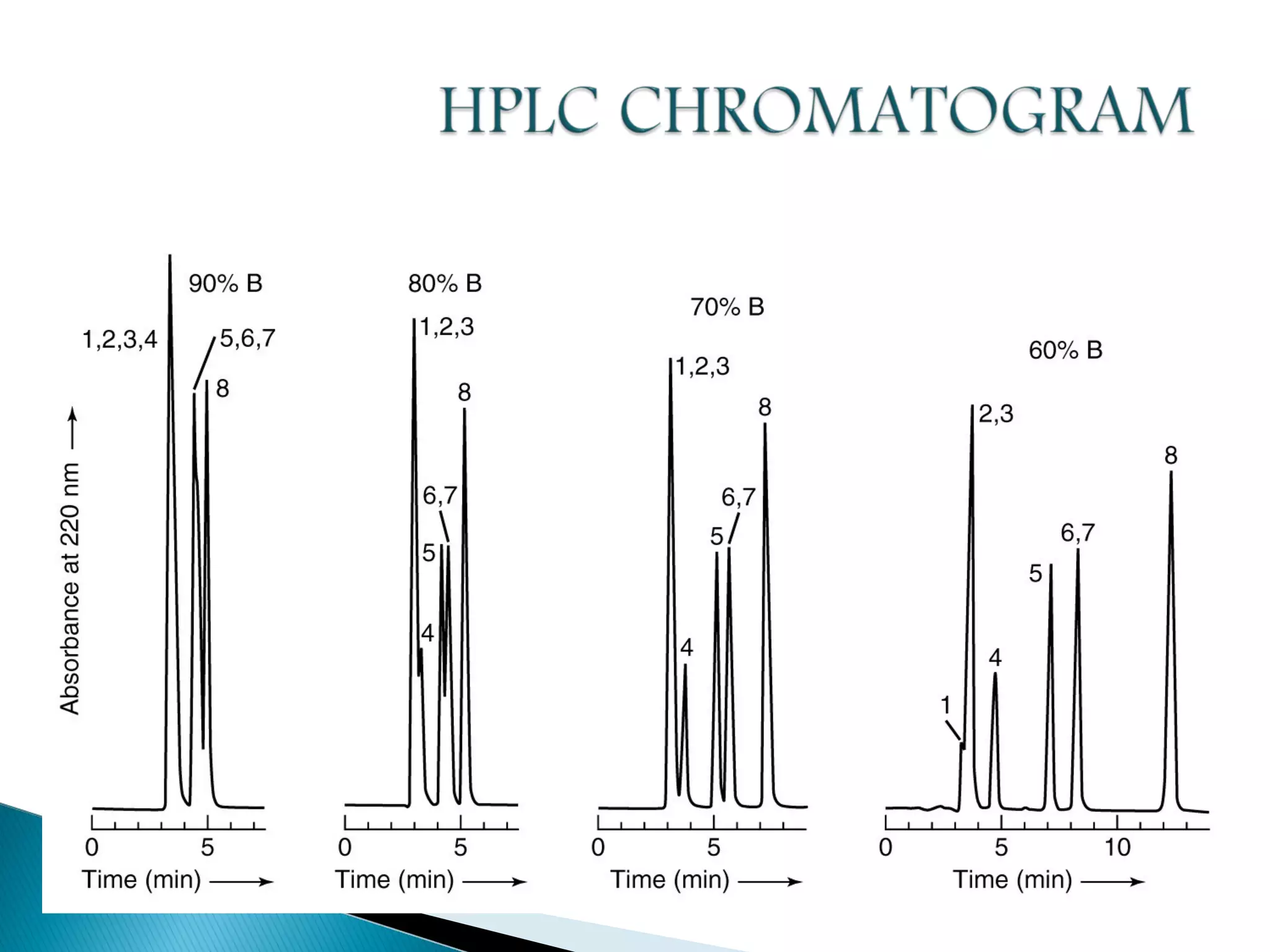
Gas chromatography is a technique used to separate mixtures by exploiting differences in how compounds interact with a mobile phase (carrier gas) and a stationary phase. Samples are injected into a column containing a stationary phase and separated components emerge from the column over time, detected, and recorded as peaks. Common applications include analyzing volatile organic compounds, flavors, fragrances, and petrochemicals. Limitations include only being able to analyze volatile compounds.